
Association between pre-operative sagittal alignment and radiographic measures of decompression following cervical laminectomy: a retrospective cohort study
Introduction
Cervical spondylotic myelopathy (CSM) is the most common cause of spinal cord dysfunction in older patients, and 10% of all patients above age 55 years demonstrate clinical CSM (1). Narrowing of the spinal canal due to structural degeneration may lead to compression of the spinal cord. Associated symptoms of CSM include upper and lower limb motor and sensory dysfunction, which can lead to functional limitations and a reduced quality of life (2). Common symptoms include: neck pain/stiffness, numbness of the hands, impaired fine motor control in the upper extremities, weakness, gait impairment, as well as autonomic symptoms such as increased urinary urgency, frequency, and incontinence. Imaging findings include anterior disk-osteophyte complexes and ligamentum flavum hypertrophy posteriorly to varying degrees, that together restrict the diameter of the spinal canal and result in cord compression (3).
The main surgical treatment for CSM involves decompression. Cervical laminectomy aims to relieve cord compression and allow for greater posterior drift of the spinal cord (4). The effectiveness of this technique has been demonstrated in several studies (5,6). Some studies demonstrate that laminectomy or laminoplasty alone may not be sufficient in patients with kyphotic cervical spine deformity and most surgeons opt for spinal fusion to minimize dynamic compression or cord injury, particularly in kyphotic spines (7-10). Thus, laminectomy with fusion has become increasingly popular over time (11). There is data showing that the incidence of post-laminectomy kyphosis is also lower following instrumented fusion of the affected spinal levels, however, there seems to be no clinical-radiologic correlation given that patients who develop post-operative kyphosis often do not progress to clinical myelopathy (12,13). Therefore, in patients without preoperative kyphotic cervical spine sagittal alignment, stand-alone laminectomy may offer acceptably low rates of post-operative kyphosis. Tashjian et al. found no correlation between the preoperative cervical spine sagittal alignment in the form of the C2–C7 Cobb angle and posterior drift of the spinal cord (14). However, without posterior drift of the spinal cord, the clinical improvement seen after laminectomy remains unexplained. We hypothesized that there is spinal cord drift that the use of ratios by Tashjian et al. did not capture, so we used direct measurement of decompression by assessing the change in the cord width and CSF space anterior and posterior to the cord pre-operatively and post-operatively. Fehlings et al. have already reported on the relationship between sagittal alignment of the cervical spine and functional outcomes in patients with CSM undergoing laminectomy through the use of validate tools including modified Japanese Orthopedic Association scores, Nurick grade, Neck Disabilty Index, and Short-Form 36v2 (SF36v2) (15). Although previous studies have studied the correlation between pre-operative cervical alignment and spinal cord drift, none have looked at the association of pre-operative neck curvature with surgical outcomes using specific quantitative radiographic measures used in this study (14,16). Furthermore, Kimura et al. hypothesized that tethering of the spinal cord in kyphotic spines would limit cord drift following laminectomies (9). Therefore, we stratified our patients into kyphotic (negative Cobb angle) and straight/lordotic (positive Cobb angle) groups and assessed whether there is less cord drift in the former group following laminectomy, which would support the tethering hypothesis proposed previously.
Another important consideration for surgical management of CSM is the number of levels that can be safely decompressed without leading to sagittal instability. It has been previously shown that spinal stability is negatively affected with an increasing number of levels resected (17).
The primary objective of this study was to determine the association between pre-operative cervical sagittal alignment and the extent of radiographic post-operative cord decompression in patients undergoing laminectomy for CSM. Secondary objectives were to determine the effect of laminectomy on the change in cervical spinal sagittal alignment, whether the number of levels decompressed correlates with the amount of space created for the compressed spinal cord or a change in the cervical spine’s alignment, and the effect of laminectomy on spinal cord signal abnormalities. Given the differences in biomechanics of the craniovertebral junction (C1 and C2 levels) and the levels below, we also did a subgroup analysis evaluating the impact on alignment in those who had laminectomy at C1 and/or C2 and those who did not.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jss-21-41).
Methods
Design
This study was conducted as a retrospective case series of patients undergoing cervical surgical decompression between 2015 and 2020 by one fellowship-trained Orthopaedic Spine surgeon in an academic institution. The study was approved by the Research Ethics Board at our institution prior to enrolment of patients. The primary outcomes of the study were the differences between the width of the spinal cord and the surrounding CSF space in millimeters pre- and post-operatively. The secondary outcomes were the status of spinal cord signal abnormality on MRI, the number of levels decompressed, and the change in C2–C7 Cobb angle.
Participants
Patients who had undergone laminectomy at one or more cervical levels between the years 2015 and 2020 as a treatment for signs and symptoms of CSM were included in this study. All of the patients in this study underwent posterior bilateral decompression. These patients were contacted by phone and if they had not already had a post-op MRI, they were invited to our study and given a requisition for an MRI scan.
Radiographic measurements
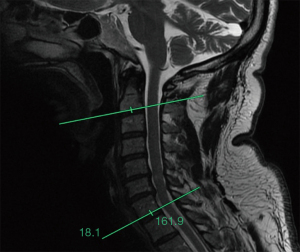
Radiological analysis involved comparison of pre- and post-operative magnetic resonance imaging (MRI) scans for all patients. Although standing radiographs are important in assessing sagittal alignment in surgical planning of CSM patients, we opted to use MRI scans for comparison to other studies reporting on spinal cord drift, which have used this method (14,18-22). XERO Viewer and picture archiving and communication system (PACS) image reviewing services were used to access the MRI scans. The ruler and angle measurement tools on these imaging services were used to measure the amount of C2–C7 sagittal alignment in the form of the mid-sagittal Cobb angle. Two lines were drawn to measure this angle with the first one being parallel to the terminal plate of C2 and the second parallel to the terminal plate of C7. The angle between these two was measured (Figure 1) (23-25). The difference in the amount of room that the surgery provided for the spinal cord was measured in two ways: (I) the change in the width of their spinal cord and (II) the amount of surrounding cerebrospinal fluid (CSF) in millimeters pre-operatively and post-operatively at the decompressed levels. All measurements were conducted three times and an average of the three measurements was reported in results. All measurements were made by the first author who was not involved in the treatment of the patients included in the study. Persistence of signal abnormalities in the spinal cord using T1/T2-weighted images was also documented (26).
Statistical analysis
Data were entered into an Excel spreadsheet designed for the study and imported into International Business Machines Corporation (IBM) Statistical Package for the Social Sciences (SPSS) Version 25 for Windows (Armonk, New York, 2018) for statistical analysis. Data were initially analyzed descriptively, including means, standard deviations and medians for continuous data, and frequencies and percentages for categorical data. The underlying distributions of the continuous data were tested for normality using the Shapiro-Wilk test (27). Pre- and post-operative data were compared using the Paired Samples t-tests and the Wilcoxon Signed Ranks test with a P value of <0.05 considered to be statistically significant. Patients with missing post-operative MRI scans were excluded from the analyses.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Kingston General Hospital and individual consent for this retrospective analysis was waived.
Results
Baseline characteristics
Review of the patient database yielded 37 patients who met criteria for enrollment in the study. At the time of publication, two patients did not have post-op MRI data and were excluded from the study, leaving us with a sample size of 35 patients. There were 25 male (71.4%) and 10 female (28.6%) patients who underwent a multi-level cervical laminectomy by a single Orthopaedic spine surgeon for CSM treatment during the study period. Patient age ranged from 33 to 83 years old with a mean of 65.29±10.98 years old (Table 1).
Table 1
| Baseline characteristics | N=35 |
|---|---|
| Age | |
| Mean ± SD (years) | 65.29±10.78 |
| Median (years) | 67 |
| Sex | |
| Female | 28.6% [10] |
| Male | 71.4% [25] |
| ASA | |
| 2 | 17.1% [6] |
| 3 | 71.4% [25] |
| 4 | 8.6% [3] |
| Unknown | 2.9% [1] |
| Comorbidities | |
| Cardiovascular disease | 28.6% [10] |
| Respiratory disease | 20% [7] |
ASA, American Society of Anaesthesiologists’ classification of Physical Health.
Intra-operative and post-operative variables
The number of levels decompressed were as follows: 15 patients (42.9%) underwent laminectomy at four levels, 13 patients (37.1%) at three levels, 4 patients (11.4%) at five levels, and 2 patients (5.7%) at two levels, and 1 patient at 6 levels (2.9%). None of the patients had any intra-operative complications and none were readmitted within 30 days of the procedure. Two patients had subsequent decompression surgery for recurrent symptoms nine and 54 months following the index procedure (Table 2). The average follow-up time for all patients following surgery was 27.21±14.93 months (range, 4–41; median, 28; IQR, 19.25). The average time between the pre-operative and post-operative MRI scans was 24.33±12.66 months (range, 5–46; median, 20; IQR, 21) and the average time from surgery to post-operative MRI scan was 20.44±13.18 months (range, 3–39, median, 18.5; IQR, 23.5).
Table 2
| Variables of interest | N=35 |
|---|---|
| Number of levels decompressed | |
| 2 | 5.7% [2] |
| 3 | 37.1% [13] |
| 4 | 42.9% [15] |
| 5 | 11.4% [4] |
| 6 | 2.9% [1] |
| Intraoperative complications† | |
| Yes | 0% [0] |
| No | 100% [35] |
| Estimated blood loss (mL), mean ± SD | 135.81±150.23 |
| 30-day readmission | |
| Yes | 0% [0] |
| No | 100% [35] |
| Repeat surgery‡ | |
| Yes | 5.7% [2] |
| No | 94.3% [33] |
†, intraoperative complications include intraoperative blood transfusion and dural tears requiring surgical repair; ‡, for persistent symptoms of CSM in the area decompressed during index surgery.
Changes in the spinal cord and surrounding structures after surgery
There were improvements in all parameters, including increased space in front of the cord (mean =1.28 mm; 95% confidence interval 0.95–1.61; P<0.001), behind the cord (mean =1.72 mm; 95% confidence interval 1.39–2.05; P<0.001), and width of the cord itself (mean difference =1.06 mm; 95% confidence interval 0.73–1.37; P<0.001), which corresponds with drift of the cord (Table 3).
Table 3
| Variable | Mean (mm) | Standard deviation | Standard error mean |
|---|---|---|---|
| Change in front (most severe level) | 2.06 | 1.31 | 0.221 |
| Change in front (average of all levels) | 1.28 | 0.961 | 0.163 |
| Change in back (most severe level) | 3.20 | 1.35 | 0.228 |
| Change in back (average of all levels) | 1.72 | 0.965 | 0.163 |
| Change in spinal cord (most severe level) | 2.17 | 1.65 | 0.280 |
| Change in spinal cord (average of all levels) | 1.07 | 0.910 | 0.154 |
Association between pre-operative kyphotic sagittal alignment and post-operative parameters
The average pre-operative cervical Cobb Angle was 6.05°±14.17° and the average post-operative Cobb angle was 3.15°±16.64°. Thirty one percent of patients (n=11) had pre-operative cervical kyphotic sagittal alignment, while 40% (n=14) had cervical kyphotic sagittal alignment at the time of follow up MRI scans. The change in Cobb angle following laminectomy was −2.89°±7.80°. Though not statistically significant, there was a weak negative correlation between more lordotic pre-op Cobb angles and the change in sagittal alignment following surgery (Figure 2, correlation coefficient −0.171, P=0.327). There was a moderate negative correlation between the degree of pre-operative kyphotic sagittal alignment and increase in CSF space in front of the cord (correlation coefficient −0.337, P=0.048) and an increase in the width of the cord itself (correlation coefficient −0.388, P=0.021). However, there was no statistically significant association between the pre-operative degree of kyphotic sagittal alignment and CSF space behind the cord (Table 4).

Table 4
| Spearman’s Rho | Average change behind cord | Average change in front of cord | Average change in cord width |
|---|---|---|---|
| Pre-op C2–C7 Cobb | |||
| Correlation coefficient | 0.213 | 0.337* | 0.388* |
| Sig. (2-tailed) | 0.220 | 0.048 | 0.021 |
| N | 35 | 35 | 35 |
*, correlation is significant at the 0.05 level (2-tailed).
Effect of increasing number of levels decompressed and post-operative cervical alignment
The majority of patients underwent either a three- or four-level laminectomy. Small, non-statistically significant positive correlations were found between increasing levels of laminectomy and space in-front of the cord and cord width (correlation coefficients 0.220, 0.278, P values =0.205, 0.106, respectively). There was no statistically significant association between increasing levels of decompression and post-operative change in cervical sagittal alignment (P=0.546) (Table 5). The subgroup analysis showed that the mean change in C2–C7 Cobb angle for patients who had C1 and/or C2 laminectomy (n=12) was −4.58°, and the change for those who did not have C1 or C2 was −2.01°. This difference was not statistically significant (P=0.363).
Table 5
| Spearman’s Rho | Average change behind cord | Average change in front of cord | Average change in cord width | Change in C2–C7 cobb angle |
|---|---|---|---|---|
| Number of levels | ||||
| Correlation coefficient | −0.261 | 0.220 | 0.278 | 0.106 |
| Sig. (2-tailed) | 0.130 | 0.205 | 0.106 | 0.546 |
| N | 35 | 35 | 35 | 35 |
Comparison of pre-operative and post-operative spinal cord signal abnormality
Spinal cord signal abnormality was assessed using T1/T2 weighted images from pre-operative and post-operative scans and any areas of hyperintensity were noted. Spinal cord signal abnormality of MRI persisted post-operatively in those with pre-existing signal abnormality and was not improved with laminectomy (P=1.00).
Discussion
Several studies have already demonstrated strong evidence for the effectiveness of laminectomy in patients presenting with significant neurologic symptoms and dysfunction secondary to CSM (5,6). The benefit of cervical laminectomy in patients with pre-existing cervical kyphotic deformity has been questioned previously and studies have reported laminectomy to be less effective in this population (7-10). These studies have involved clinical correlates to determine effectiveness. Our study aimed to assess the effectiveness of laminectomy in a sample of 35 patients with varying cervical spinal alignments through a quantitative approach that has not been previously employed.
We found that laminectomy created more CSF space in front of and behind the cord and allowed increased width of the cord itself. This quantitative improvement in the spinal canal parameters shows that there is some degree of improvement in the stenotic area of the cervical spine following laminectomy, regardless of pre-operative alignment. As previously reported, the mechanism behind laminectomy is increased spinal cord drift following surgery, which can result in improvement of neurological symptoms present in CSM (4). The results in the present study are consistent with this mechanism as we found increased spinal cord drift in all cases, regardless of the pre-operative sagittal alignment in the form of the C2–C7 Cobb angle.
There is data suggesting that laminectomy alone may not be sufficient to improve symptoms in patients with spinal instability and a high degree of pre-operative kyphosis, resulting in insufficient posterior drift of the spinal cord and increased incidence of post-operative kyphosis (8,28). Additionally, Shamji et al. have shown that patients with a lordotic preoperative cervical spine sagittal alignment have better myelopathy improvements than kyphotic patients (29). It is understood that increasing kyphotic curvature of the cervical spine leads to pathological changes such as cord tethering and flattening of small feeder vessels, especially on the anterior side of the spinal canal, which is directly exposed to the mechanical compression (30,31). Given these findings, there has been concern from some surgeons regarding the benefit of cervical laminectomy in kyphotic patients as the spinal cord is tethered by the brain superiorly and the filum terminale inferiorly, therefore in theory, limiting how much it can shift back post-operatively in the presence of kyphosis (32). Previous studies have shown consistent results regarding the correlation between pre-operative sagittal alignment and clinical outcome (33). However, Tashjian et al. found no correlation between pre-operative sagittal alignment and the spinal cord drift following surgery (14). Our study showed that the pre-operative severity of kyphotic sagittal alignment of the cervical spine was associated with less space created in-front of the cord and less change in spinal cord width after cervical laminectomy. However, we did not find a significant correlation between kyphotic alignment and space created behind the spinal cord, meaning that pre-operative cervical spine alignment did not affect how much CSF space the procedure was able to create posteriorly. Based on these results, it seems that a more kyphotic sagittal alignment may limit the amount of space laminectomy can create anterior to the spinal cord and does not significantly affect the space created posteriorly, which is consistent with the aforementioned anatomical explanation for concerns associated with effectiveness of laminectomy in patients with pre-operative kyphosis. With tethering and a kyphotic cervical spine, we found that the spinal cord drift was lower following surgery compared to a lordotic cervical spine. This result does not support the findings previously reported by Tashjian et al. as the pre-operative sagittal alignment did have an effect on the spinal cord drift when measured directly.
Previous studies have shown that increasing number of levels involved in laminectomy can lead to vertebral instability (18). However, an increasing number of levels fused in an anterior approach is correlated with increasing complication risk, which has led to the general preference for a posterior approach if three or more levels are pathologic (34). We did not find a significant association between the number of levels decompressed and change in cervical spine alignment. The change in alignment was not significantly different between those who had C1 and/or C2 laminectomy and those who had neither level involved. Despite not being statistically significant, this result is consistent with previous literature that reports greater destabilization in cervical spine sagittal alignment with involvement of the C1 and C2 levels (35).
We found that spinal cord signal abnormality persisted in those with pre-existing signal abnormality, meaning that surgery has no effect on the already degenerated segments of the spinal cord. It should be noted that we only considered the presence, and not the degree, of signal abnormality. These findings are of important clinical significance as cord signal abnormalities, when noted by primary care physicians post-operatively, can lead to unnecessary referrals to orthopedic surgery or neurosurgery. Our results support the permanence of spinal cord degeneration as evidenced by persisting spinal cord signal abnormality on post-operative MRI scans.
Strengths and limitations
Previous literature has demonstrated inferior clinical outcomes in CSM patients with pre-operative cervical spine kyphotic sagittal alignment. Our study extended this further by evaluating the effects of pre-operative cervical spine alignment on radiographic parameters within the spinal canal and the effect of increasing levels of decompression on cervical spine stability using the same radiographic parameters. Since the electronic measurements on the imaging viewers used in this study were made by a single individual, there is the possibility of user error, which may affect reliability of the results. However, each measurement was repeated three times and the average was taken to limit errors in measurement. All procedures were performed by a single surgeon in one academic setting and there was a range of 6 months to 4 years of follow-up time from pre-operative to post-operative MRI scans. It would be valuable to assess the replicability of results in different settings, stratified by follow up time. The small sample size limited the power of the analyses and may have resulted in a considerable margin of error. This would limit our ability to generalize treatment guidelines based on our results. Additionally, some patients had very short follow-up time and the study did not assess the relationship between cervical spine alignment and post-operative functional outcomes. Lastly, although osteoporosis is a risk factor for post-laminectomy kyphosis, we had too few patients to determine its effect on our outcomes.
Conclusions
A pre-operative kyphotic deformity limits the amount of spinal cord drift and space laminectomy is able to create in the spinal canal. Laminectomy does not resolve pre-existing spinal cord signal abnormality on MRI. Increasing number of levels decompressed do not appear to cause cervical spine instability, nor do they offer additional improvement in the space created in the spinal canal by the procedure. Further studies are needed to validate these results on a larger scale.
Acknowledgments
Preliminary work was presented at the William Ersil Resident Research Day Conference 2019 in Kingston, ON, Canada. The work has been presented at the 2021 Canadian Spine Society annual scientific meeting.
Funding: McLaughlin Summer Studentship ($6,000) for summer 2019.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jss-21-41
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jss-21-41
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jss-21-41). HA received the McLaughlin Summer Research Studentship ($6,000) at Queen’s University, Kingston, Ontario, Canada in summer 2019 for this project. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Kingston General Hospital and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Klineberg E. Cervical spondylotic myelopathy: a review of the evidence. Orthop Clin North Am 2010;41:193-202. [Crossref] [PubMed]
- Oh T, Lafage R, Lafage V, et al. Comparing Quality of Life in Cervical Spondylotic Myelopathy with Other Chronic Debilitating Diseases Using the Short Form Survey 36-Health Survey. World Neurosurg 2017;106:699-706. [Crossref] [PubMed]
- Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist 2013;19:409-21. [Crossref] [PubMed]
- Epstein NE. Laminectomy for cervical myelopathy. Spinal Cord 2003;41:317-27. [Crossref] [PubMed]
- Geck MJ, Eismont FJ. Surgical options for the treatment of cervical spondylotic myelopathy. Orthop Clin North Am 2002;33:329-48. [Crossref] [PubMed]
- Edwards CC 2nd, Heller JG, Murakami H. Corpectomy versus laminoplasty for multilevel cervical myelopathy: an independent matched-cohort analysis. Spine (Phila Pa 1976) 2002;27:1168-75. [Crossref] [PubMed]
- Winestone JS, Farley CW, Curt BA, et al. Laminectomy, durotomy, and piotomy effects on spinal cord intramedullary pressure in severe cervical and thoracic kyphotic deformity: a cadaveric study. J Neurosurg Spine 2012;16:195-200. [Crossref] [PubMed]
- Fujiyoshi T, Yamazaki M, Kawabe J, et al. A new concept for making decisions regarding the surgical approach for cervical ossification of the posterior longitudinal ligament: the K-line. Spine (Phila Pa 1976) 2008;33:E990-3. [Crossref] [PubMed]
- Kimura I, Shingu H, Nasu Y. Long-term follow-up of cervical spondylotic myelopathy treated by canal-expansive laminoplasty. J Bone Joint Surg Br 1995;77:956-61. [Crossref] [PubMed]
- Manzano GR, Casella G, Wang MY, et al. A prospective, randomized trial comparing expansile cervical laminoplasty and cervical laminectomy and fusion for multilevel cervical myelopathy. Neurosurgery 2012;70:264-77. [Crossref] [PubMed]
- Han K, Lu C, Li J, et al. Surgical treatment of cervical kyphosis. Eur Spine J 2011;20:523-36. [Crossref] [PubMed]
- Kim BS, Dhillon RS. Cervical Laminectomy With or Without Lateral Mass Instrumentation: A Comparison of Outcomes. Clin Spine Surg 2019;32:226-32. [Crossref] [PubMed]
- Buell TJ, Buchholz AL, Quinn JC, et al. Importance of Sagittal Alignment of the Cervical Spine in the Management of Degenerative Cervical Myelopathy. Neurosurg Clin N Am 2018;29:69-82. [Crossref] [PubMed]
- Tashjian VS, Kohan E, McArthur DL, et al. The relationship between preoperative cervical alignment and postoperative spinal cord drift after decompressive laminectomy and arthrodesis for cervical spondylotic myelopathy. Surg Neurol 2009;72:112-7. [Crossref] [PubMed]
- Fehlings MG, Santaguida C, Tetreault L, et al. Laminectomy and fusion versus laminoplasty for the treatment of degenerative cervical myelopathy: results from the AOSpine North America and International prospective multicenter studies. Spine J 2017;17:102-8. [Crossref] [PubMed]
- Kaptain GJ, Simmons NE, Replogle RE, et al. Incidence and outcome of kyphotic deformity following laminectomy for cervical spondylotic myelopathy. J Neurosurg 2000;93:199-204. [PubMed]
- Katsumi Y, Honma T, Nakamura T. Analysis of cervical instability resulting from laminectomies for removal of spinal cord tumor. Spine (Phila Pa 1976) 1989;14:1171-6. [Crossref] [PubMed]
- Boudreau C, Carrondo Cottin S, Ruel-Laliberté J, et al. Correlation of supine MRI and standing radiographs for cervical sagittal balance in myelopathy patients: a cross-sectional study. Eur Spine J 2021;30:1521-8. [Crossref] [PubMed]
- Ross MN, Ross DA. Minimally Invasive Cervical Laminectomy for Cervical Spondylotic Myelopathy. Clin Spine Surg 2018;31:331-8. [Crossref] [PubMed]
- Gembruch O, Jabbarli R, Rashidi A, et al. Surgery for Degenerative Cervical Myelopathy: What Really Counts? Spine (Phila Pa 1976) 2021;46:294-9. [Crossref] [PubMed]
- Radcliff KE, Limthongkul W, Kepler CK, et al. Cervical laminectomy width and spinal cord drift are risk factors for postoperative C5 palsy. J Spinal Disord Tech 2014;27:86-92. [Crossref] [PubMed]
- Ashana AO, Cohen JR, Evans B, et al. Regression of Anterior Disk-Osteophyte Complex Following Cervical Laminectomy and Fusion for Cervical Spondylotic Myelopathy. Clin Spine Surg 2017;30:E609-14. [Crossref] [PubMed]
- Cobb Angle. In: Encyclopedia of Diagnostic Imaging 2008.
- Harrison DE, Harrison DD, Cailliet R, et al. Cobb method or Harrison posterior tangent method: which to choose for lateral cervical radiographic analysis. Spine (Phila Pa 1976) 2000;25:2072-8. [Crossref] [PubMed]
- Xing R, Liu W, Li X, et al. Characteristics of cervical sagittal parameters in healthy cervical spine adults and patients with cervical disc degeneration. BMC Musculoskelet Disord 2018;19:37. [Crossref] [PubMed]
- Al-Mefty O, Harkey LH, Middleton TH, et al. Myelopathic cervical spondylotic lesions demonstrated by magnetic resonance imaging. J Neurosurg 1988;68:217-22. [Crossref] [PubMed]
- Lewis-Beck M, Bryman A, Futing Liao T. Shapiro-Wilk Test. Sage 2012;
- Abduljabbar FH, Teles AR, Bokhari R, et al. Laminectomy with or Without Fusion to Manage Degenerative Cervical Myelopathy. Neurosurg Clin N Am 2018;29:91-105. [Crossref] [PubMed]
- Shamji MF, Mohanty C, Massicotte EM, et al. The Association of Cervical Spine Alignment with Neurologic Recovery in a Prospective Cohort of Patients with Surgical Myelopathy: Analysis of a Series of 124 Cases. World Neurosurg 2016;86:112-9. [Crossref] [PubMed]
- Ramchandran S, Protopsaltis TS, Sciubba D, et al. Prospective multi-centric evaluation of upper cervical and infra-cervical sagittal compensatory alignment in patients with adult cervical deformity. Eur Spine J 2018;27:416-25. [Crossref] [PubMed]
- Shimizu K, Nakamura M, Nishikawa Y, et al. Spinal kyphosis causes demyelination and neuronal loss in the spinal cord: a new model of kyphotic deformity using juvenile Japanese small game fowls. Spine (Phila Pa 1976) 2005;30:2388-92. [Crossref] [PubMed]
- Bakhsheshian J, Mehta VA, Liu JC. Current Diagnosis and Management of Cervical Spondylotic Myelopathy. Global Spine J 2017;7:572-86. [Crossref] [PubMed]
- Löfgren H, Osman A, Blomqvist A, et al. Sagittal Alignment After Laminectomy Without Fusion as Treatment for Cervical Spondylotic Myelopathy: Follow-up of Minimum 4 Years Postoperatively. Global Spine J 2020;10:425-32. [Crossref] [PubMed]
- Wilson JR, Tetreault LA, Kim J, et al. State of the Art in Degenerative Cervical Myelopathy: An Update on Current Clinical Evidence. Neurosurgery 2017;80:S33-45. [Crossref] [PubMed]
- Wang WX, Zhao YB, Lu XD, et al. Influence of extending expansive open-door laminoplasty to C1 and C2 on cervical sagittal parameters. BMC Musculoskelet Disord 2020;21:75. [Crossref] [PubMed]


