Etiology and treatment of cervical kyphosis: state of the art review—a narrative review
Introduction
Cervical spine kyphosis causes neck pain, myelopathy, radiculopathy, problems with horizontal gaze, swallowing and breathing. Treating cervical spine kyphosis presents challenges, as vital structures such as the vertebral artery, trachea, and esophagus, are within close proximity. The cervical spine also has various functions including supporting the weight of the head, protecting neurovascular structures, maintaining horizontal gaze, and allowing a wide range of motion. Although cervical spine kyphosis has been increasingly common due to the growing elderly population, there is a paucity of evidence on the management of cervical kyphosis. We aimed to provide a state-of-the-art review to help clinicians to comprehensively understand the management of cervical kyphosis.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://dx.doi.org/10.21037/jss-21-54).
Methods
The available literature relevant to cervical kyphosis was reviewed. PubMed, Medline, OVID, EMBASE, and Cochrane were used to review the literature. Only English articles published between 1965 and 2020 were included.
Discussion/summary
Biomechanics
Anterior elements consist of vertebral bodies and intervertebral discs, resisting compression and bearing approximately 36% of the axial load. Posterior elements consist of the facets, laminae, spinous processes, posterior musculature, and ligamentous structures. Posterior elements bear the remaining 64% of the axial load under lordosis and function as a tension band (1). Normal functioning of these anterior and posterior structures plays an important role, as a lordotic alignment can resist large compressive loads and minimize stress on the vertebral endplates (2,3) preventing cervical pain and loss of function.
In the healthy cervical spine, axial loads are applied along the instantaneous axis of rotation (IAR). The loads are supported along the anterior column of the spine. With aging of spine, the disc continues to lose height and lordosis is reduced. The axial force is offset from the IAR, producing a greater moment arm at the point of rotation. Greater loss of lordosis increases the moment arm, worsening kyphotic deformity (4).
Etiology
There are multiple causes of cervical kyphosis including degeneration, infection, trauma, inflammation, and iatrogenic. Post-laminectomy kyphosis, degenerative kyphosis, kyphosis secondary to ankylosing spondylitis are highlighted in this article as they are more common than kyphosis from trauma, infection, neoplasm, and congenital anomalies. Post-laminectomy kyphosis is the most common cause of iatrogenic kyphosis, and may lead to neurologic deficits. Ankylosing spondylitis bears particular mention since after relatively minor trauma, patients may develop instability leading to post traumatic kyphosis.
Post-laminectomy kyphosis
Post-laminectomy kyphosis develops gradually with disruption of posterior tension band, increasing compressive loads on the anterior elements. Once the cervical spine becomes kyphotic, the weight of the head is shifted forward, and the increased anterior axial load may lead to further kyphosis (Figure 1). In adults, the incidence of postoperative kyphosis after multi-level laminectomy has been reported to be 21% (5). There is a higher incidence of post-laminectomy kyphosis in children, 37% to 100% (6-8), because the anterior vertebral bodies in children are less ossified and composed of cartilaginous portion, leading to wedging under cumulative stress (8).
Risk factors for post-laminectomy kyphosis include preoperative loss of cervical lordosis (CL) (5), resection of the bony elements, facet capsule destruction (9-12), loss of the posterior ligamentous structures and irradiation. Posterior facet joint disruption has a significant impact on cervical spine biomechanics as the posterior column supports 64% of cervical load (1). Thus, careful attention must be paid to preserving the facets during laminectomy. Irradiation has been associated with higher postoperative kyphosis rates in children. Mayfield reviewed 74 patients undergoing radiotherapy for neuroblastoma and reported a 76% incidence of cervical kyphosis after an average 13-year follow-up with 20% of survivors requiring correction surgery (13).
Some surgeons prefer laminotomy (14), laminoplasty (15,16) or minimally invasive decompression (17,18) to avoid post-operative kyphosis. Laminoplasty is the most widely accepted method and has achieved improved clinical outcomes without decreasing CL and without adding significant operative time and morbidity compared with laminectomy and fusion (19). However, even laminoplasty or minimally invasive decompression can cause postoperative kyphosis in the absence of neurological disease, when the facet joint capsules are disrupted, when excessive facet joint is resected (20-22), or when the semispinalis cervicis muscle is not reattached to C2 properly (23). Other iatrogenic causes include collapse of adjacent discs following multilevel discectomies, especially without a concurrent fusion (24).
Degenerative kyphosis
Degenerative changes in the cervical spine, involving multiple levels, may result in cervical kyphosis (Figure 2). Common causes including a potential genetic pre-disposition, repetitive trauma, and smoking (24). As the aging of spine, CL is reduced. The axial force is offset from the IAR, causing a greater moment arm at the point of rotation. Without restoration of lordosis and load balance along the cervical spine, further axial loading will induce further progression of the kyphosis.
Often, there is an underappreciation of global sagittal balance where there is concurrent, excessive thoracic kyphosis or following a long fusion into the upper thoracic spine that develops proximal junctional kyphosis (PJK) and an excessive T1 slope (T1S) that is non-physiological. The loss of disc height following degeneration typically starts anteriorly within the disc, which leads to further axial load in the anterior column. This change in cervical spine biomechanics causes vertebral body wedging, leading to subsequent kyphosis.
Ankylosing spondylitis
Ankylosing spondylitis is seronegative spondyloarthropathy with known association with the major histocompatibility complex antigen HLA-B27 (25). The typical deformity is a combination of a thoracic hyperkyphosis and loss of lumbar lordosis with the forward shift of the head. This deformity progresses, resulting in fixed sagittal imbalance. Cervical spine typically shows cervical and/or cervicothoracic junction kyphosis (a chin-on-chest deformity: Figure 3), leading to difficulty with horizontal gaze, swallowing, chewing, ambulation, laying flat, and activities of daily living. Patients with ankylosing spondylitis also tend to have osteoporosis as a result of stress shielding (26). In addition, the spine in ankylosing spondylitis loses flexibility and acts like a long bone, becoming susceptible to major injury even with minor forces (27,28). Consequently, post traumatic cervical kyphosis, with or without translation, may develop following minor trauma resulting in the development of an acute cervical kyphotic deformity and progressive neurologic injury.
Clinical presentation
The most common symptom is neck fatigue and pain. Neck pain results from degeneration of the discs and facet joints, or increased stress on the posterior soft tissue and musculature in an attempt to hold the head in a biomechanical advantageous position. The head needs to be perfectly centered over the thorax or within pelvic “cone of balance” or the mechanical imbalance severely stresses the circumferential cervical musculature resulting in fatigue, imbalance, increased energy expenditure and muscle strain (29). Cervical kyphosis is often associated with myelopathy and/or radiculopathy as well. A severe kyphosis can cause problems with appearance, horizontal gaze, swallowing and breathing.
Radiographic evaluation
Standard evaluation includes static and dynamic cervical radiographs. Standing anteroposterior and lateral 36-inch radiographs are also useful to assess global spinal alignment (30-34) as a close relationship exists between the cervical and thoracolumbar spine. Cervical kyphosis may be primary or reciprocal due to thoracolumbar deformity. Thoracic kyphosis and thoracolumbar alignment directly affect the cervical alignment to maintain horizontal gaze (35). Furthermore, 53% of adult patients with a thoracolumbar deformity have a concomitant cervical deformity (36). It is optimal to obtain both dedicated cervical and 36-inch radiographs because clavicle position caused a decrease in the T1S (37,38). Computed tomography scans with reconstructions and magnetic resonance imaging (MRI) are also mandatory for patients undergoing corrective surgery, as they provide more detailed information about bony structures, neural elements and stenosis, vertebral artery anatomy, areas of fusion mass or previous laminectomy, and the presence of pseudarthrosis.
Radiographic measurements allow surgeons to evaluate the extent of deformity and set goals for operative correction. Comprehensive measurements, including CL, C2–7 sagittal vertical axis (SVA), chin-brow to vertical angle (CBVA), T1S, should be evaluated during surgical planning (31). Unlike spinopelvic parameters for thoracolumbar deformity, radiographic parameters in the cervical spine that affect health-related quality of life (HRQOL) have not been well defined (39,40). However, there are a few studies that have shown parameters associated with HRQOL. Oe et al. investigated cervical alignment in volunteers aged over 50 and demonstrated that C2–7 SVA, T1S, and T1S-CL negatively influenced EQ-5D (41). Hyun et al. showed that C2–7 SVA >40.8 and 70.6 mm, and T1S-CL >20° and 25° were associated with moderate and severe disability following multilevel posterior cervical fusion surgery, respectively (42). A C2–7 SVA >40 mm was reported to be correlated with increased disability in patients undergoing posterior cervical fusion (43). Ajello et al. showed that a C2–7 SVA <25 mm and CL/C7 slope >0.7 were correlated with positive outcomes following anterior cervical arthrodesis (44).
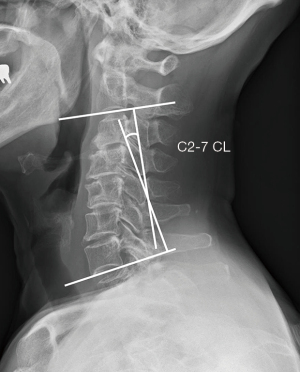
CL is measured by the angle between perpendicular lines drawn along the C2 and C7 inferior endplates (Figure 4). Previous studies showed that normal CL measured from C2–7 ranged from 10.5° to 20.3° (45-47). Harrison posterior tangent method is another measurement method for CL that may have less standard error of measurement, in which CL is measured by the angle between tangents drawn at the posterior body margins of C2 and C7 (48). Surgeons should move towards better measurement methods as the literature changes.
High cervical angle is also commonly used, which is measured by the angle between the McGregor line and the lower C2 endplate. This angle has an average value of 15.81° and work with CL inversely (49).
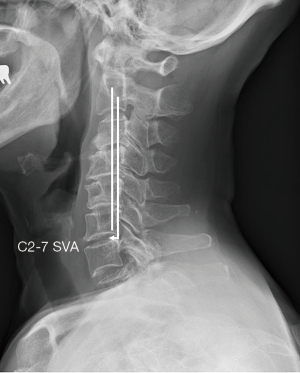
The C2–7 SVA is used as an index of regional sagittal alignment of the cervical spine. C2–7 SVA is defined as the distance between the plumb line falling from the centroid of C2 and vertical line drawn from posterior superior endplate of C7 (Figure 5). Iyer et al. examined cervical alignment in 115 asymptomatic patients from upright radiographs obtained from EOS imaging system and showed a mean C2–7 SVA of 21.3 mm (45).
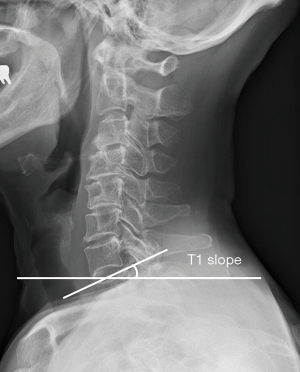
The T1S, the angle between the T1 upper end plate and the horizontal plane (Figure 6) is influenced by the alignment of thoracolumbar spine, pelvis and lower extremity. The T1S determines the amount of CL required to maintain the center of gravity of the head in a balanced position and ranges from 21° to 37°, being higher in older patients (30,41). Similarly, thoracic inlet angle (TIA) is another patient-specific parameter to predict physiological alignment of the cervical spine. TIA is measured by the angle subtended by a line drawn perpendicular through the center of the superior endplate of T1 and a line from the midpoint of the superior endplate of T1 to the apex of the manubrium (41-43,50).
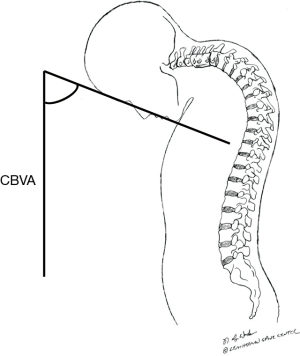
CBVA is an indirect measure of horizontal gaze and can be measured from clinical photographs or full-body EOS X-rays between the chin-brow line and the vertical line (Figure 7). A CBVA is positive when the head is tilted downwards. Lafage et al. report that a CBVA between −4.8° and +17.7° correlated with less disability (51), while Suk et al. found that the correction of CBVA between −10° and 10° had better horizontal gaze (52) in patients with ankylosing spondylitis patients undergoing pedicle subtraction osteotomy (PSO) for kyphotic deformity. Song et al. showed a postoperative CVBA between 10° and 20° had higher satisfaction with good visual field related quality of life and subjective appearance (53). These reports imply correction to neutral or a slight downward head tilt may achieve the optimal clinical outcome.
Classification
There has not been a standardized classification for cervical spinal deformity. Ames et al. developed a novel cervical deformity classification using a modified Delphi approach in 2015 (54). This classification includes a deformity descriptor and 5 modifiers. A deformity descriptor provides a basic grouping of the deformity consisting of five types; C (cervical), CT (cervicothoracic), T (thoracic), S (coronal cervical deformity), and CVJ (cranio-vertebral junction deformity). The first three types are selected based on the apex of the cervical deformity. Type S is a deformity with a C2–7 coronal Cobb angle ≥15°. Type CVJ represents primary cranio-vertebral junction deformities. The five modifiers include C2–7 SVA, horizontal gaze (CBVA), T1S-CL, myelopathy based on modified Japanese Orthopaedic Association score, and SRS-Schwab classification for thoracolumbar deformity.
Non-surgical treatment
Non-surgical treatment is indicated for mild cervical kyphosis and patients who cannot have surgery. There is a scarce evidence in the effectiveness of conservative treatment for cervical kyphosis. Moustafa et al. conducted a randomized control trial to examine if Denneroll cervical traction (Denneroll Industries, Sydney, Australia; http://www.denneroll.com) improved cervical kyphosis. After 10 weeks of the intervention, Denneroll cervical traction improved cervical alignment and the improvement was maintained until one year follow-up (55). Further study is necessary to validate.
Surgical treatment
The procedure or combination of procedures selected for correction of cervical kyphosis depends on the degree of kyphosis, the rigidity of the deformity and the quality of the bone. Specific surgical techniques include anterior cervical discectomy and fusion (ACDF), anterior cervical corpectomy and fusion (ACCF), anterior osteotomy (ATO), Smith-Petersen osteotomy (SPO), PSO, or any combination of these techniques. The goals of surgical treatment include correction of deformity, decompression of the neural elements, and spinal stabilization with solid bony fusion while avoiding complications.
Surgical planning
Surgical planning depends on multiple factors: (I) symptom pattern; (II) neurologic status and presence of neural compression; (III) etiology of the deformity; (IV) location, flexibility and characteristics of the deformity; (V) previous surgeries; (VI) presence of ankylosis and/or a fusion mass; (VII) presence of degenerative changes at the proximal/distal end vertebral levels and the cervicothoracic junction; (VIII) patient medical comorbidities; (IX) vertebral artery anatomy (especially at C1 & C2) and (X) bone quality.
The amount of correction should be assessed using the radiographic parameters that were described above. No universal normal range is determined, however, current available evidence suggests that T1S-C2–7 lordosis <15°, C2–7 SVA <40 mm, CBVA between –10° and +20° are generally acceptable ranges (45,52-54). Based on this information, the approach (anterior, posterior, or combined) and type of osteotomy can be chosen.
Decision making process
The most important goal is to know how much correction is obtained through each procedure. On average, single level ACDF, SPO, PSO, and ATO yield 3°–5°, 10°, 35°, and 17° of lordosis correction, respectively (56,57). PSO is the most powerful correction tool, however, a recent study showed that an ATO with SPOs provided equal or better corrections than a PSO with shorter hospital stay, less neurological injury and blood loss (57,58).
If anterior compressive pathology such as an osteophytic bar or calcified disc is present, then an anterior approach is typically necessary to achieve adequate decompression. If the integrity of the anterior column is compromised, such as infection or tumor cases, then anterior reconstruction is also required.
Next, the flexibility of the deformity should be assessed on dynamic X-rays. If the cervical spine is flexible and the kyphosis is corrected on extension without anterior compressive pathology, a posterior-only approach may be possible. If the deformity is rigid, anterior and/or posterior ankylosis should be evaluated using computed tomography scan. If the facets are not fused, anterior-only correction may be possible, although posterior augmentation may be necessary in case of multilevel procedures (anterior-posterior approach). If the facets and anterior column are fused, a posterior release is necessary prior to anterior correction, followed by posterior instrumentation and fusion with or without additional osteotomy depending on the extent of correction needed (posterior-anterior-posterior approach). In severe and rigid kyphotic cases, intraoperative traction may be necessary. It is recommended to use Gardner-Wells tongs with bivector traction to allow for adjusting the neck position in either flexion or extension during surgery (59).
The location of kyphosis is also important. A focal kyphosis in the mid-cervical spine often can be corrected via anterior corpectomy. If a severe focal kyphosis is present at the cervicothoracic junction, as often seen in the case with ankylosing spondylitis, a C7 or T1 PSO may be performed to correct the focal kyphosis either at the junction or below at T3.
Anterior approaches
ACDF/ACCF
ACDF is effective in restoring lordosis in cases without ankylosed facets. A single-level ACDF improved the segmental CL by 6.45°, and the overall C2–7 CL by 3.46° at 1 year (56). Multi-level ACDF is generally more effective than a single long corpectomy in restoring lordosis, as multi-level cervical discectomy provides multiple distraction points.
After standard exposure, anterior release, including disc and osteophytes, is performed. It is important to resect the uncinate processes to maximize deformity correction. After release and distraction, lordotic cages or grafts are placed at each level. A plate is placed anteriorly to the cervical spine. To generate additional correction, the plate can be contoured into the desired lordosis although this technique requires good bone quality.
Patients with severe kyphosis or ventral spinal cord compression posterior to the vertebral body may require a corpectomy to achieve adequate decompression and clear visualization of the spinal canal. Manual cervical distraction or distraction pins facilitate the placement of a structural bone graft or a cage into the corpectomy site.
Multi-level ACDFs can be an effective tool in restoring CL. However, the rate of pseudoarthrosis is relatively high ranging from 2.5% to 44% (60,61). Retrospective review of two- and three-level corpectomies with strut graft and anterior plate fixation showed failure rates of 6–9% and 50–71%, respectively (62,63). Thus, additional posterior fixation is required to facilitate fusion in multi-level cases as well as osteoporotic cases. Hybrid corpectomy and discectomy may be more advantageous than multi-level corpectomy to decrease the risk of pseudarthrosis and instrumentation failure as this procedure provides additional fixation points of the anterior plate but clinical superiority has yet to be demonstrated (64).
ATO
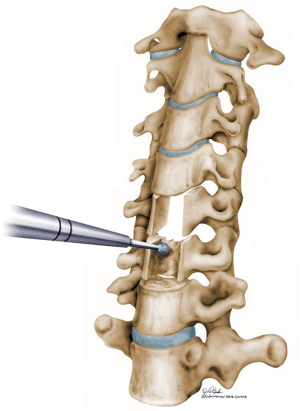
In a rigid deformity, it is sometimes hard to identify the disc space, and lateral dissection and identification of uncovertebral joint can help identify the former disc space (Figure 8). Once the former disc space is identified, Caspar distraction pins are placed perpendicular to the anterior plane above and below the levels of the resection to aid in generating lordosis. The entire width of the original disc space is taken down using high-speed burr. Bony resection is performed posteriorly to the level of the posterolateral ligament with the burr. Lateral bony resection is performed with caution to avoid vertebral artery injury.
Once the osteotomy is complete, kyphosis correction is done by using a vertebral spreader and pressing on the forehead. A structural graft is placed in the anterior half of the disc space followed by anterior plate with fixed-angle screws. If additional posterior correction is necessary, a buttress plate. Alternatively, a dynamic cervical plate, which allows for translation, may be used to hold the graft(s) in place.
Posterior approaches
Posterior cervical column osteotomy (SPO)
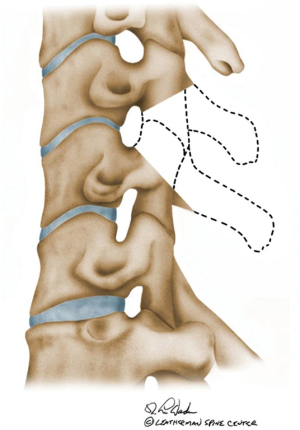
Single level SPO can yield about 10° of lordosis but require resecting both the superior and inferior articulating facets along with removal of the ligamentum flavum, lamina, and spinous processes (Figure 9). A posterior cervical column osteotomy (SPO) allows posterior-only correction but requires anterior disc mobility, preferably of near normal height to obtain kyphosis correction by pivoting the bodies through the mobile disc. In general, multi-level posterior column osteotomies (SPO) are needed to obtain the desired correction, which increases the risk of pseudarthrosis. Iatrogenic nerve root compression can occur during correction if the bony resection is inappropriate.
PSO
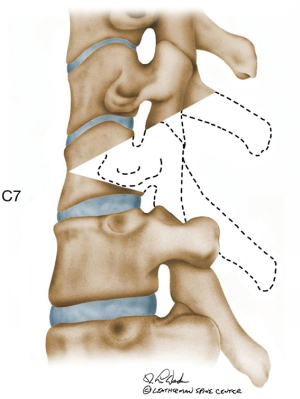
A cervical PSO is a powerful tool for deformity correction and a single level PSO provides about 35° of lordosis (Figure 10). A cervical PSO is technically demanding and may be indicated in severe fixed cervical kyphotic deformities. A cervical PSO is generally performed at C7 or below for several reasons: (I) the vertebral artery is anterior to the C7 transverse process; (II) spinal canal is larger and mobility of the spinal cord at C7–T1 is higher; (III) C8 nerve root injury does not cause a catastrophic outcome in hand function. Of note, vertebral artery goes through the C7 foramen transversarium in up to 5% of the patients. Therefore, it is critical to carefully review the preoperative images to check for any vascular anomaly. In that case, a PSO should be performed at T1.
Bone graft options
A structural bone graft (iliac crest or fibula) or cages can be used for structural support and should be wide enough to span from uncinate to uncinate. Structural grafts should be taller anteriorly than posteriorly and allow for 1–2 mm of subsidence. If subsequent posterior correction is necessary, the surgeon should make sure that the posterior aspect of the endplates behind the graft are not touching.
Several graft options can be used for structural cages made of PEEK (poly-ethyl-ethyl ketone) or titanium. Morselized iliac crest autograft or allograft, local autograft, and recombinant human bone morphogenetic protein 2 (BMP-2) can be placed in the cage. BMP-2 has been associated with inflammatory reactions and airway obstruction when used in the anterior cervical spine and should be used with caution.
Complications
Smith et al. report that the overall early complication rate was 43.6%. The most common complications included dysphagia (11.5%), deep wound infection (6.4%), C5 palsy (6.4%), and respiratory failure (5.1%). Early complication rates were significantly different based on surgical approach: anterior-only (27.3%), posterior-only (68.4%), and anterior-posterior/posterior-anterior-posterior (79.3%) (65). Smith et al. reported all-cause mortality at a mean of 1.2 years following surgery was 9.2% (66). Similarly, Etame et al. reviewed the literature on outcome after cervical deformity surgery (67). They reported mortality rate of 2.3%, major medical complication rate of 3.3%, and the rate of neurological complication rate of 13.5% (67). They also reported complication rates as dysphagia (2.5%), hoarseness due to vocal cord paralysis (3.4%), and need for tracheostomy (1.8%) or gastrostomy tube (2.3%), durotomy (1.0%), and pseudarthrosis (3.8%).
The most catastrophic complication is neurological injury. The overall rate of neurological injury is approximately 23% after cervical extension osteotomy with permanent neurologic complication rate being 4.3% (58). C8 nerve root palsy seems to be the most common neurological deficit with reported incidence of 19–38% although it is transient in most cases (68,69).
Injury to vertebral artery is rare but could happen when decompression extends lateral to the vertebral body and enter the transverse foramen or when there is an anomaly in the path. Manual pressure and hemostatic agents are used to control hemorrhage. Direct exposure and repair are suitable if possible and if required interventional radiology can stint or embolize the vertebral artery if required.
Limitations
There are several limitations in this review article. First, there are other etiologies that were not discussed here although those are not major etiologies. Universal classification has not been developed yet. In addition, surgical strategy may be at surgeons’ discretion in many instances.
Conclusions
The primary etiologies of cervical kyphosis include degenerative, infection, post-laminectomy, and ankylosing spondylitis. Cervical kyphosis can cause not only neck pain, myelopathy, and radiculopathy, but also problems with horizontal gaze, swallowing or breathing. CL, C2–7 SVA, CBVA, and T1S should be evaluated from upright lateral 36-inch film. Comprehensive preoperative planning is required, which includes (I) symptom pattern; (II) neurologic status and presence of neural compression; (III) etiology of the deformity; (IV) location, flexibility and characteristics of the deformity; (V) previous surgeries; (VI) presence of ankylosis and fusion mass; (VII) presence of degenerative changes at the proximal/distal end vertebral levels and the cervicothoracic junction; (VIII) patient medical comorbidities; (IX) vertebral artery anatomy (especially at C1 & C2) and (X) bone quality. Current treatment options include anterior discectomy and fusion, ATO, Smith-Peterson osteotomy, PSO, or a combination of these based on careful preoperative evaluation.
Our review article presents comprehensive findings on cervical kyphosis. This article would provide a broad range of current findings on cervical kyphosis and be a useful tool for clinicians.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/jss-21-54
Peer Review File: Available at https://dx.doi.org/10.21037/jss-21-54
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jss-21-54). JRD reports personal fees from Medtronic, personal fees from DePuy, other from Federation of Spine Association, other from SRS, personal fees from Pfizer, outside the submitted work. MD reports personal fees from Medtronic, personal fees from NuVasive, outside the submitted work. LYC reports personal fees from AO spine, personal fees from Center for Spine Surgery and Research of the University of Southern Denmark, personal fees from University of Louisville Institutional Review Board, grants from Scoliosis Research Society Research, grants from Orthopedic Educational Research Fund, grants from Integra, grants from Pfizer, personal fees from Pfizer, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pal GP, Sherk HH. The vertical stability of the cervical spine. Spine (Phila Pa 1976) 1988;13:447-9. [Crossref] [PubMed]
- Miura T, Panjabi MM, Cripton PA. A method to simulate in vivo cervical spine kinematics using in vitro compressive preload. Spine (Phila Pa 1976) 2002;27:43-8. [Crossref] [PubMed]
- Harrison DE, Harrison DD, Janik TJ, et al. Comparison of axial and flexural stresses in lordosis and three buckled configurations of the cervical spine. Clin Biomech (Bristol, Avon) 2001;16:276-84. [Crossref] [PubMed]
- Ferrara LA. The biomechanics of cervical spondylosis. Adv Orthop 2012;2012:493605 [Crossref] [PubMed]
- Kaptain GJ, Simmons NE, Replogle RE, et al. Incidence and outcome of kyphotic deformity following laminectomy for cervical spondylotic myelopathy. J Neurosurg 2000;93:199-204. [PubMed]
- Bell DF, Walker JL, O'Connor G, et al. Spinal deformity after multiple-level cervical laminectomy in children. Spine (Phila Pa 1976) 1994;19:406-11. [Crossref] [PubMed]
- Cattell HS, Clark GL Jr. Cervical kyphosis and instability following multiple laminectomies in children. J Bone Joint Surg Am 1967;49:713-20. [Crossref] [PubMed]
- Yasuoka S, Peterson HA, Laws ER Jr, et al. Pathogenesis and prophylaxis of postlaminectomy deformity of the spine after multiple level laminectomy: difference between children and adults. Neurosurgery 1981;9:145-52. [Crossref] [PubMed]
- McLaughlin MR, Wahlig JB, Pollack IF. Incidence of postlaminectomy kyphosis after Chiari decompression. Spine (Phila Pa 1976) 1997;22:613-7. [Crossref] [PubMed]
- Papagelopoulos PJ, Peterson HA, Ebersold MJ, et al. Spinal column deformity and instability after lumbar or thoracolumbar laminectomy for intraspinal tumors in children and young adults. Spine (Phila Pa 1976) 1997;22:442-51. [Crossref] [PubMed]
- Yeh JS, Sgouros S, Walsh AR, et al. Spinal sagittal malalignment following surgery for primary intramedullary tumours in children. Pediatr Neurosurg 2001;35:318-24. [Crossref] [PubMed]
- Katsumi Y, Honma T, Nakamura T. Analysis of cervical instability resulting from laminectomies for removal of spinal cord tumor. Spine (Phila Pa 1976) 1989;14:1171-6. [Crossref] [PubMed]
- Mayfield JK, Riseborough EJ, Jaffe N, et al. Spinal deformity in children treated for neuroblastoma. J Bone Joint Surg Am 1981;63:183-93. [Crossref] [PubMed]
- Abbott R, Feldstein N, Wisoff JH, et al. Osteoplastic laminotomy in children. Pediatr Neurosurg 1992;18:153-6. [Crossref] [PubMed]
- Hirabayashi K, Bohlman HH. Multilevel cervical spondylosis. Laminoplasty versus anterior decompression. Spine (Phila Pa 1976) 1995;20:1732-4. [Crossref] [PubMed]
- Maeda T, Arizono T, Saito T, et al. Cervical alignment, range of motion, and instability after cervical laminoplasty. Clin Orthop Relat Res 2002;132-8. [Crossref] [PubMed]
- Minamide A, Yoshida M, Simpson AK, et al. Microendoscopic laminotomy versus conventional laminoplasty for cervical spondylotic myelopathy: 5-year follow-up study. J Neurosurg Spine 2017;27:403-9. [Crossref] [PubMed]
- Nori S, Shiraishi T, Aoyama R, et al. Muscle-Preserving Selective Laminectomy Maintained the Compensatory Mechanism of Cervical Lordosis After Surgery. Spine (Phila Pa 1976) 2018;43:542-9. [Crossref] [PubMed]
- Heller JG, Edwards CC 2nd, Murakami H, et al. Laminoplasty versus laminectomy and fusion for multilevel cervical myelopathy: an independent matched cohort analysis. Spine (Phila Pa 1976) 2001;26:1330-6. [Crossref] [PubMed]
- Zdeblick TA, Abitbol JJ, Kunz DN, et al. Cervical stability after sequential capsule resection. Spine (Phila Pa 1976) 1993;18:2005-8. [Crossref] [PubMed]
- Albert TJ, Vacarro A. Postlaminectomy kyphosis. Spine (Phila Pa 1976) 1998;23:2738-45. [Crossref] [PubMed]
- Nowinski GP, Visarius H, Nolte LP, et al. A biomechanical comparison of cervical laminaplasty and cervical laminectomy with progressive facetectomy. Spine (Phila Pa 1976) 1993;18:1995-2004. [Crossref] [PubMed]
- Takeuchi K, Yokoyama T, Aburakawa S, et al. Anatomic study of the semispinalis cervicis for reattachment during laminoplasty. Clin Orthop Relat Res 2005;126-31. [Crossref] [PubMed]
- Haden N, Latimer M, Seeley HM, et al. Loss of inter-vertebral disc height after anterior cervical discectomy. Br J Neurosurg 2005;19:469-74. [Crossref] [PubMed]
- Wells LJ, Edwards JH, Webley M, et al. Ankylosing spondylitis, HLA, and BF. Lancet 1979;1:104-5. [Crossref] [PubMed]
- Bronson WD, Walker SE, Hillman LS, et al. Bone mineral density and biochemical markers of bone metabolism in ankylosing spondylitis. J Rheumatol 1998;25:929-35. [PubMed]
- Detwiler KN, Loftus CM, Godersky JC, et al. Management of cervical spine injuries in patients with ankylosing spondylitis. J Neurosurg 1990;72:210-5. [Crossref] [PubMed]
- Kanter AS, Wang MY, Mummaneni PV. A treatment algorithm for the management of cervical spine fractures and deformity in patients with ankylosing spondylitis. Neurosurg Focus 2008;24:E11 [Crossref] [PubMed]
- Dubousset J. Three-dimensional analysis of the scoliotic deformity. In: Weinstein SL. editor. The Pediatric Spine: Principles and Practice. Volume 1. Raven Press, 1994:479-96.
- Ames CP, Blondel B, Scheer JK, et al. Cervical radiographical alignment: comprehensive assessment techniques and potential importance in cervical myelopathy. Spine (Phila Pa 1976) 2013;38:S149-60. [Crossref] [PubMed]
- Scheer JK, Tang JA, Smith JS, et al. Cervical spine alignment, sagittal deformity, and clinical implications: a review. J Neurosurg Spine 2013;19:141-59. [Crossref] [PubMed]
- Smith JS, Lafage V, Ryan DJ, et al. Association of myelopathy scores with cervical sagittal balance and normalized spinal cord volume: analysis of 56 preoperative cases from the AOSpine North America Myelopathy study. Spine (Phila Pa 1976) 2013;38:S161-70. [Crossref] [PubMed]
- Smith JS, Shaffrey CI, Lafage V, et al. Spontaneous improvement of cervical alignment after correction of global sagittal balance following pedicle subtraction osteotomy. J Neurosurg Spine 2012;17:300-7. [Crossref] [PubMed]
- Ramchandran S, Smith JS, Ailon T, et al. Assessment of Impact of Long-Cassette Standing X-Rays on Surgical Planning for Cervical Pathology: An International Survey of Spine Surgeons. Neurosurgery 2016;78:717-24. [Crossref] [PubMed]
- Diebo BG, Challier V, Henry JK, et al. Predicting Cervical Alignment Required to Maintain Horizontal Gaze Based on Global Spinal Alignment. Spine (Phila Pa 1976) 2016;41:1795-800. [Crossref] [PubMed]
- Smith JS, Lafage V, Schwab FJ, et al. Prevalence and type of cervical deformity among 470 adults with thoracolumbar deformity. Spine (Phila Pa 1976) 2014;39:E1001-9. [Crossref] [PubMed]
- Park SM, Song KS, Park SH, et al. Does whole-spine lateral radiograph with clavicle positioning reflect the correct cervical sagittal alignment? Eur Spine J 2015;24:57-62. [Crossref] [PubMed]
- Carreon LY, Smith CL, Dimar JR 2nd, et al. Correlation of cervical sagittal alignment parameters on full-length spine radiographs compared with dedicated cervical radiographs. Scoliosis Spinal Disord 2016;11:12. [Crossref] [PubMed]
- Mohanty C, Massicotte EM, Fehlings MG, et al. Association of preoperative cervical spine alignment with spinal cord magnetic resonance imaging hyperintensity and myelopathy severity: analysis of a series of 124 cases. Spine (Phila Pa 1976) 2015;40:11-6. [Crossref] [PubMed]
- Shamji MF, Mohanty C, Massicotte EM, et al. The Association of Cervical Spine Alignment with Neurologic Recovery in a Prospective Cohort of Patients with Surgical Myelopathy: Analysis of a Series of 124 Cases. World Neurosurg 2016;86:112-9. [Crossref] [PubMed]
- Oe S, Togawa D, Nakai K, et al. The Influence of Age and Sex on Cervical Spinal Alignment Among Volunteers Aged Over 50. Spine (Phila Pa 1976) 2015;40:1487-94. [Crossref] [PubMed]
- Hyun SJ, Han S, Kim KJ, et al. Assessment of T1 Slope Minus Cervical Lordosis and C2-7 Sagittal Vertical Axis Criteria of a Cervical Spine Deformity Classification System Using Long-Term Follow-up Data After Multilevel Posterior Cervical Fusion Surgery. Oper Neurosurg (Hagerstown) 2019;16:20-6. [Crossref] [PubMed]
- Tang JA, Scheer JK, Smith JS, et al. The impact of standing regional cervical sagittal alignment on outcomes in posterior cervical fusion surgery. Neurosurgery 2015;76:S14-21; discussion S21. [Crossref] [PubMed]
- Ajello M, Marengo N, Pilloni G, et al. Is It Possible To Evaluate the Ideal Cervical Alignment for Each Patient Needing Surgery? An Easy Rule To Determine the Appropriate Cervical Lordosis in Preoperative Planning. World Neurosurg 2017;97:471-8. [Crossref] [PubMed]
- Iyer S, Lenke LG, Nemani VM, et al. Variations in Occipitocervical and Cervicothoracic Alignment Parameters Based on Age: A Prospective Study of Asymptomatic Volunteers Using Full-Body Radiographs. Spine (Phila Pa 1976) 2016;41:1837-44. [Crossref] [PubMed]
- Gore DR, Sepic SB, Gardner GM. Roentgenographic findings of the cervical spine in asymptomatic people. Spine (Phila Pa 1976) 1986;11:521-4. [Crossref] [PubMed]
- Takeshita K, Murakami M, Kobayashi A, et al. Relationship between cervical curvature index (Ishihara) and cervical spine angle (C2--7). J Orthop Sci 2001;6:223-6. [Crossref] [PubMed]
- Harrison DE, Harrison DD, Cailliet R, et al. Cobb method or Harrison posterior tangent method: which to choose for lateral cervical radiographic analysis. Spine (Phila Pa 1976) 2000;25:2072-8. [Crossref] [PubMed]
- Le Huec JC, Thompson W, Mohsinaly Y, et al. Sagittal balance of the spine. Eur Spine J 2019;28:1889-905. [Crossref] [PubMed]
- Lee SH, Kim KT, Seo EM, et al. The influence of thoracic inlet alignment on the craniocervical sagittal balance in asymptomatic adults. J Spinal Disord Tech 2012;25:E41-7. [Crossref] [PubMed]
- Lafage R, Challier V, Liabaud B, et al. Natural Head Posture in the Setting of Sagittal Spinal Deformity: Validation of Chin-Brow Vertical Angle, Slope of Line of Sight, and McGregor's Slope With Health-Related Quality of Life. Neurosurgery 2016;79:108-15. [Crossref] [PubMed]
- Suk KS, Kim KT, Lee SH, et al. Significance of chin-brow vertical angle in correction of kyphotic deformity of ankylosing spondylitis patients. Spine (Phila Pa 1976) 2003;28:2001-5. [Crossref] [PubMed]
- Song K, Su X, Zhang Y, et al. Optimal chin-brow vertical angle for sagittal visual fields in ankylosing spondylitis kyphosis. Eur Spine J 2016;25:2596-604. [Crossref] [PubMed]
- Ames CP, Smith JS, Eastlack R, et al. Reliability assessment of a novel cervical spine deformity classification system. J Neurosurg Spine 2015;23:673-83. [Crossref] [PubMed]
- Moustafa IM, Diab AA, Hegazy F, et al. Does improvement towards a normal cervical sagittal configuration aid in the management of cervical myofascial pain syndrome: a 1- year randomized controlled trial. BMC Musculoskelet Disord 2018;19:396. [Crossref] [PubMed]
- Gillis CC, Kaszuba MC, Traynelis VC. Cervical radiographic parameters in 1- and 2-level anterior cervical discectomy and fusion. J Neurosurg Spine 2016;25:421-9. [Crossref] [PubMed]
- Kim HJ, Piyaskulkaew C, Riew KD. Comparison of Smith-Petersen osteotomy versus pedicle subtraction osteotomy versus anterior-posterior osteotomy types for the correction of cervical spine deformities. Spine (Phila Pa 1976) 2015;40:143-6. [Crossref] [PubMed]
- Etame AB, Than KD, Wang AC, et al. Surgical management of symptomatic cervical or cervicothoracic kyphosis due to ankylosing spondylitis. Spine (Phila Pa 1976) 2008;33:E559-64. [Crossref] [PubMed]
- Tan LA, Riew KD, Traynelis VC. Cervical Spine Deformity-Part 3: Posterior Techniques, Clinical Outcome, and Complications. Neurosurgery 2017;81:893-8. [Crossref] [PubMed]
- Samartzis D, Shen FH, Matthews DK, et al. Comparison of allograft to autograft in multilevel anterior cervical discectomy and fusion with rigid plate fixation. Spine J 2003;3:451-9. [Crossref] [PubMed]
- Emery SE, Fisher JR, Bohlman HH. Three-level anterior cervical discectomy and fusion: radiographic and clinical results. Spine (Phila Pa 1976) 1997;22:2622-4; discussion 2625. [Crossref] [PubMed]
- Vaccaro AR, Falatyn SP, Scuderi GJ, et al. Early failure of long segment anterior cervical plate fixation. J Spinal Disord 1998;11:410-5. [Crossref] [PubMed]
- Sasso RC, Ruggiero RA Jr, Reilly TM, et al. Early reconstruction failures after multilevel cervical corpectomy. Spine (Phila Pa 1976) 2003;28:140-2. [Crossref] [PubMed]
- Steinmetz MP, Stewart TJ, Kager CD, et al. Cervical deformity correction. Neurosurgery 2007;60:S90-S97. [Crossref] [PubMed]
- Smith JS, Ramchandran S, Lafage V, et al. Prospective Multicenter Assessment of Early Complication Rates Associated With Adult Cervical Deformity Surgery in 78 Patients. Neurosurgery 2016;79:378-88. [Crossref] [PubMed]
- Smith JS, Shaffrey CI, Kim HJ, et al. Prospective Multicenter Assessment of All-Cause Mortality Following Surgery for Adult Cervical Deformity. Neurosurgery 2018;83:1277-85. [Crossref] [PubMed]
- Etame AB, Wang AC, Than KD, et al. Outcomes after surgery for cervical spine deformity: review of the literature. Neurosurg Focus 2010;28:E14 [Crossref] [PubMed]
- Tokala DP, Lam KS, Freeman BJ, et al. C7 decancellisation closing wedge osteotomy for the correction of fixed cervico-thoracic kyphosis. Eur Spine J 2007;16:1471-8. [Crossref] [PubMed]
- Langeloo DD, Journee HL, Pavlov PW, et al. Cervical osteotomy in ankylosing spondylitis: evaluation of new developments. Eur Spine J 2006;15:493-500. [Crossref] [PubMed]