
Is there consensus on the perioperative management of Xa inhibitors in patients undergoing elective spine surgery?—A survey of current spine surgeon practices
Introduction
Venous thromboembolism (VTE) incidence following elective spine surgery can range from 0.3% to 31%. Even at the lowest incidence rates, VTEs represent a serious postoperative risk (1). For patients with a history of thromboembolic conditions, the risk of perioperative thrombotic event is higher than the general population and perioperative anticoagulation management poses a challenge (2). A balance must be struck between preventing another VTE and causing a bleeding complication of surgery. The North American Spine Society’s Evidence-Based Clinical Guideline notes that the safety and efficacy of chemoprophylaxis in spine surgery is countered by the risk of bleeding complications and thus timing of anticoagulant use is controversial—offering no definitive guideline for surgical practice (3). In fact, to the authors’ knowledge, the best practice for management of chronic anticoagulation medications perioperative to elective spine surgery marks a current gap in the literature. In situations such as this, a poll of active spine surgeons who have earned membership into spine surgery societies offer the opportunity to obtain a cross-section of current practice that can then inform future directed clinical research on the subject.
Among the anticoagulants used for chronic management of VTE and prophylaxis for other common conditions, such as atrial fibrillation, Xa inhibitors (Xai) are the newest class of medication. Likely due to the standard oral dosing regimen and no need for laboratory monitoring, Xai use has become more and more prevalent. Xai work by inhibiting Xa which reduces thrombin and prevents the formation of clots. Common Xai medications include rivaroxaban, apixaban, and edoxaban. Considering their cost-effectiveness, ease of use, and equivalent, or even superior, prevention of thromboembolic events and all-cause mortality, it can be expected that the usage of Xai will continue to grow. This rising prevalence has introduced a new variable for surgeons to consider (4-6). An international consensus statement by the European Association of Cardiothoracic Anaesthesiology recommended Xai be held for 2–4 days prior to surgery, while other guidelines offer a range of 1 to 5 days depending on factors, such as anti-factor Xa plasma level assay availability (6-8). The variance among these recommendations reflect an attempt to standardize guidelines based on the half-life of Xai’s similar to their currently approved dosing regimens, while at the same time allowing for some manner of individualization based on a patient’s risk both for a thrombotic and a bleeding event.
Adding to this uncertainty is the absence of an affordable, widely available laboratory assessment for Xai activity. Though Anti-Factor Xa Assays have been created, they are not widely accessible. More commonly available tests such as prothrombin time (PT) and activated partial thromboplastin time (aPTT) are not sensitive (8,9). Additionally, the lack of a readily available anticoagulant reversal agent increases the difficulty in decision making when using these drugs (10). Without clear guidelines, a widely available test to directly measure Xai activity, nor a cost-effective reversal agent, it is unclear how spine surgeons are currently striking their own balance in regards to perioperative Xai management. The purpose of this study is to capture current practice trends regarding the perioperative management of Xai and other anticoagulation medications among spine surgeons.
We present the following article in accordance with the SURGE reporting checklist (available at https://dx.doi.org/10.21037/jss-20-637).
Methods
This study and its components were reviewed and approved by Mayo Clinic Institutional Review Board (Registration No. IRB00000020). Subject’s participation in the study was entirely voluntary with consent to participate received upon subject’s submission of survey answers. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). An electronic survey was sent through SurveyMonkey® to all surgeon members of AOSpine North America, Cervical Spine Research Society (CSRS), and Lumbar Spine Research Society (LSRS). Responses were submitted anonymously. All responses were voluntary and respondents were not compensated for completing the survey. The surveys were sent out to the society members twice, with reminders, before being closed to further responses.
The survey was comprised of 11 questions (Table 1) that were designed to characterize the medical training of the respondent, their current practice regarding perioperative anticoagulants, and discuss future options for perioperative anticoagulant management. The survey asked questions related to their pre- and post-operative practices with patients who were taking Xai or other chronic anticoagulants. Questions were all single-best option, multiple-choice. A 100% response rate was required for each survey submitted.
Table 1
| # | Question |
|---|---|
| 1 | Please describe your training background? |
| 2 | Approximately how many years have you been practicing Spine Surgery? |
| 3 | Please describe your practice? |
| 4 | How long do your medical colleagues recommend holding direct factor Xa inhibitors pre-operatively (e.g., 2 days means 2 full days off inhibitors prior to date of surgery)? |
| 5 | How long do you personally require patients to remain off of their direct factor Xa inhibitor pre-operatively (e.g., 2 days means 2 full days off inhibitors prior to date of surgery)? |
| 6 | On average how long do you hold your patients direct factor Xa inhibitor post-operatively (e.g., 2 days means no anticoagulation on the day of surgery plus one full day)? |
| 7 | In general how long do you hold all chemical anticoagulation post-operatively (e.g., 2 days means no anticoagulation on the day of surgery plus one full day)? |
| 8 | Have you noted a change in the rate of perioperative bleeding complications (epidural hematoma, wound drainage, etc.) in your patients taking direct factor Xa inhibitors, when compared to other chemical anticoagulants (i.e., warfarin, heparin, LMWH, etc.)? |
| 9 | If readily available and the cost is acceptable, would you routinely obtain a laboratory study to determine if your patient has cleared their direct factor Xa inhibitor prior to surgery? |
| 10 | If the proposed factor Xa inhibitor test demonstrated active metabolites of the direct factor Xa inhibitor, would you consider canceling or postponing surgery? |
| 11 | Does a patient’s pre-operative stroke risk assessment (CHADS2 score) factor into how you will hold anticoagulation post-operatively? |
Statistical analysis
Statistics were performed utilizing JMP Pro 14 (SAS Institute Inc., Cary, NC, USA). Univariate comparisons of respondent demographics, recommended and reported practices, and reported bleeding complications were conducted. Wilcoxon Rank-Sum tests compared continuous variables while Chi-square and Fisher exact tests compared categorical variables. Alpha level was set to P<0.05 for significance.
Results
Demographics
There was a total of 116 unique completed surveys submitted, all of which were included in analysis. Of the 116 respondents, 26 (22.4%) were neurosurgeons and 90 (77.6%) were orthopedic surgeons. Demographics of the respondents, by practice, are illustrated in Table 2.
Table 2
| Practice | 1–2 years (%) | 3–10 years (%) | 11–20 years (%) | >20 years (%) | Total |
|---|---|---|---|---|---|
| Academic Neurosurgery | 2 (1.7) | 6 (5.2) | 5 (4.3) | 7 (6.0) | 20 (17.2) |
| Private Neurosurgery | 1 (0.9) | 1 (0.9) | 1 (0.9) | 3 (2.6) | 6 (5.2) |
| Academic Orthopedic Surgery | 4 (3.4) | 14 (12.1) | 14 (12.1) | 17 (14.7) | 49 (42.6) |
| Private Orthopedic Surgery | 5 (4.3) | 15 (12.9) | 7 (6.0) | 14 (12.1) | 41 (35.3) |
| Total | 12 (10.3) | 36 (31.0) | 27 (23.3) | 41 (35.3) | 116 (100.0) |
Pre-operative practices regarding Xai
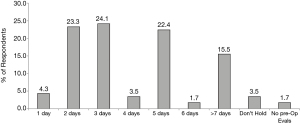
The recommended pre-operative window for holding Xai provided to respondents by their medical colleagues varied widely from 0 to >7 days. Among all respondents, 73.28% reported that their medical colleagues recommended holding Direct Factor Xai preoperatively for anywhere between 2–5 days. The most commonly recommended Xai preoperative holds were 3 days (24.1%), 2 days (23.3%), and 5 days (22.41%) (Figure 1).
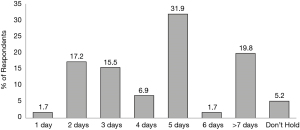
Similar to the recommended pre-operative hold length, 71.56% of respondents reported that they personally require patients to remain off of their Direct Factor Xai preoperatively for anywhere between 2–5 days. The most commonly used preoperative Xai holds were 5 days (31.9%), >7 days (19.8%), and 2 days (17.2%) (Figure 2).
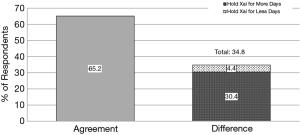
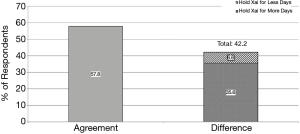
Though there was a similar quantity of respondents recommending 2–5 days of Xai hold, only 65.2% of respondents reported that their practice was in agreement with the recommendations of their medical colleagues (P≤0.0001). Of the 41.2% whose practice was different than recommended by colleagues, 35.6% recommended a hold of greater length, while 5.6% recommended a shorter hold length than what their medical colleagues recommended (Figure 3). Among orthopedic surgeons, there was a greater disagreement between recommendation and practice with 57.8% of respondents practicing in agreement with recommendations, 35.6% requiring a longer Xai hold and 6.6% requiring a shorter Xai hold (Figure 4).
When compared by specialty and years of experience, there was no distinguishable trend, or preference, when it came to the chosen number of days for pre-operative Xai hold.
Post-operative practices regarding Xai
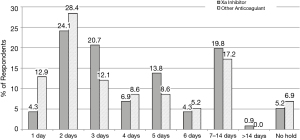
Respondents were asked about their post-operative practices with regards to both patients on Xai and patients who were on any anticoagulants (Figure 5). Postoperative Xai practices varied widely, with an overall trend toward longer holds than that of other anticoagulants. The three most frequently used lengths of hold for Xai were 2 days (24.14%), 3 days (20.69%), and 7–14 days (19.83%). The three most frequently used hold lengths for patients on any other anticoagulant were 2 days (28.45%), 7–14 days (17.24%), and 1 day (12.93%). 66.38% of respondents held Xai for 3 or more days postoperatively. When asked about holding other anticoagulants for 3 or more days postoperatively, the percentage of respondents dropped to 51.72%.
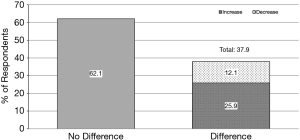
Individual responses revealed that 37.93% of respondents showed differences within their own practice between length of Xai hold and length of holding other anticoagulants (Figure 6) (P=0.0125). of respondents who had a difference in their postoperative practices, 68.2% held Xai longer than other anticoagulants, while 31.8% held Xai for a shorter postoperative period.
Opportunities for rational consensus and future clinical research
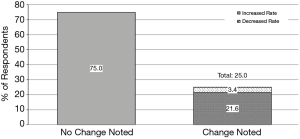
One out of four respondents reported noticing a change in the rate of perioperative bleeding complications among patients who used Xai (Figure 7). Of those who noticed a change, 86.2% reported an increase and 13.8% reported a decrease. However, those who reported noticing an increase in bleeding complications only accounted for 39% of those who held direct Xai longer than other anticoagulants preoperatively. Further, only 44% of those who reported noticing an increase in bleeding complications for Xai patients described a difference in their practice between Xai and other anticoagulant holds following surgery.
When respondents were asked if they would routinely use an easily accessible and affordable lab test that would measure Xai clearance, 80.2% of respondents said yes. If the proposed test existed, and they had a patient who had Xai test results indicating on-going activity of the drug, 96.55% of respondents reported they would cancel or postpone surgery for one of four reasons. These four reasons were: (I) yes, unequivocally; (II) yes, but only if the patient’s other coagulation tests were abnormal or scientific evidence demonstrated heightened bleeding risk; (III) yes, but only if the patient’s other coagulation tests were also abnormal; (IV) yes, but only if there is scientific evidence demonstrating heightened bleeding risk.
Discussion
This survey reveals that there is a vast range of practice surrounding perioperative Xai management and a clear need for evidence based direction on management as Xai use continues to grow. There exists a wide range of recommended and practiced chronic anticoagulant hold lengths in elective spine surgery with no clear preference. Most surgeons appear to rely upon the recommendations from their medical colleagues regarding preoperative holding length for Xai. However, there were discrepancies between the medical consultant recommended hold length and the practiced hold length, particularly among Orthopedic surgeons, who were more likely to opt for a pre-operative hold longer than what was recommended by a medical consultant. This inconsistency may highlight conflicting perspectives of risk aversion between the medical doctor and surgeon in chronic anticoagulant use—thrombotic events vs. postoperative hemorrhage/hematoma. Conversely, patients who require long term Xai usually have comorbidities that place them at the highest risk for perioperative thrombotic events, further complicating the medical decision making. The novelty of Xai leaves surgeons with little evidence for differences in outcomes for Xai patients, let alone the impact of premature or delayed holds or resumption, with which to make practice decisions. The true rate of adverse postoperative events in chronic Xai users is unclear. Unfortunately, there is little evidence regarding the use of Xai in orthopaedic surgery, outside of arthroplasty surgery where there is significant information on the topic (11,12). Even among the arthroplasty patient population there is little consensus on the optimal perioperative management of anticoagulation. A survey of the American Association of Hip and Knee Surgeons (AAHKS) membership revealed wide variability in practice patterns, highlighting the need for ongoing research (12).
One of the most interesting findings in this study was that 25% of respondents reported observing changes in postoperative bleeding complications for patients on Xai, yet the length of Xai hold times did not reflect the surgeon’s experience with postoperative bleeding complications. In fact, more than half of those who held Xai longer than other anticoagulants postoperatively did not report noticing an increase in postoperative bleeding complications. The cause of this trend was not informed by this study, though it may be related to perceived risks and tendency towards risk aversion in the setting of a lack of availability of Xai level and reversal agents. The rapid onset of activity of Xais may also have been a factor in decision making for when to restart the medication, since other anticoagulants take much longer to become effective than the Xais. Additionally, the literature has conflicting evidence regarding differences in bleeding risk between Xai and more conventional chemoprophylaxis agents (13).
Currently, the literature suggests that there are significant variations in bleeding risk demonstrated in patients taking Xais. In one study, fondaparinux and rivaroxaban were shown to increase the risk of major/clinically relevant non-major (CRNM) bleeding compared to E40 and increase the incidence of major/CRNM bleeding when compared to a variety of enoxaparin doses. However, in the same study, apixaban had a lower risk than enoxaparin for major/CRNM bleeding (14). There is also evidence that bleeding risk for a single Xai varies by site—further confounding the process of perioperative Xai management. In a study comparing rivaroxaban versus warfarin, rates of bleeding were similar across all three bleeding classification types: major bleeding, major or CRNM bleeding, and CRNM bleeding. Yet, there was higher incidence of major GI bleeding among the rivaroxaban population than those on warfarin (15,16). There is limited literature specifically addressing rates of bleeding complications at the spine or following spinal surgery. Beyond considering the Xai, or the rate of bleeding complications across sites, there is also variation in bleeding complication rates across dosage. Edoxaban 30 mg has been shown to have an increased rate of Major or CRNM bleeding when compared to warfarin, while edoxaban 60 mg was shown to have a decreased rate of Major or CRNM bleeding, but a significantly increased rate of major GI bleeding (17,18). When comparing the rate of major postoperative bleeding across rivaroxaban doses in patients who underwent THA, there was a significant dose trend. Those at the lowest and top three dosing tiers had higher incidences of major postoperative bleeding than patients on enoxaparin (19). A dose-response meta-analysis of both enoxaparin and Xai dosing also demonstrated the complex balance between efficacy and risk of major bleeding complication in use of apixaban, edoxaban, rivaroxaban, and dabigatran following orthopedic surgeries (20). Other studies on rivaroxaban in arthroplasty surgery suggest rivaroxaban incurs no more significant rates of major bleeding than enoxaparin, despite having increased rates of bleeding complications (21,22). Importantly, the relative prevalence of studies surrounding rivaroxaban in the orthopedic setting highlights the scarcity of literature on bleeding risks and complications for other Xais. Overall, this plethora of conflicting and contingent literature, without a comprehensive summary, leaves spinal surgeons without a clear understanding to guide perioperative Xai management.
The desire for more information regarding the optimal perioperative management of Xai is clear. Xai testing appealed to over 80.2% of respondents. If such a test was scientifically substantiated, our results indicate that surgeons would almost unanimously use it and patient’s care would be directly influenced by it. However, the practicality and utility of such tests would still depend upon further research into the correlation between Xai activity levels and perioperative bleeding and thrombotic risk. A key limitation with current tests is the high cost and limited availability of these tests. Increased Xai use will likely necessitate better availability of tests and reversal agents, and clear guidelines.
There are several limitations inherent to survey methodology. This survey is representative of those who were willing to voluntarily complete the series of questions. As a multiple choice format, only a limited number of choices can be presented which may not accurately represent the full breadth of clinical scenarios or capture the nuances of these complex medical treatment decisions. Additionally, this survey is not meant to reflect the relative effectiveness of or endorse any particular treatment strategy. Rather, it was meant only to illustrate the current practice strategies among spine surgeons today. Despite these limitations, this survey does help to demonstrate the ongoing controversies and lack of consensus among surgeons and physicians regarding the use of direct factor Xai for perioperative anticoagulation in spine surgery.
Conclusions
In summary, there continues to be wide variability in the perioperative use of Xai among spine surgeons. The need for clear guidelines regarding the use of these medications is of increasing importance as the use of these medications becomes increasingly common. As testing for Xai levels becomes more available, surgeons are likely to incorporate that data into their clinical practice, which will impact patient care. Management of Xai and prevention of thromboembolism is a particularly challenging task that should be well informed. Research into the current practices of spine surgeons would be further supported by increased survey participation and further questions regarding the perceived risks and advantages to increased or decreased perioperative Xai holds. The lack of established risks and guidelines for spine surgical patients taking Xai should be addressed with further research into Xai patient outcomes and optimal perioperative Xai hold length.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the SURGE reporting checklist. Available at https://dx.doi.org/10.21037/jss-20-637
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jss-20-637
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jss-20-637). Dr. BC receives grants from AO Spine NA Institutional Fellowship support, royalties or licenses from DePuy Synthes, Zimmer Biomet, and Wolters Kluwer, consulting fees from Surgalign, patents with DePuy Synthes and Zimmer Biomet, and Stock with Spinology and Tenex. Dr. BE receives grants or contracts from NIH NIAMS, Stryker Spine, and SI Bone, consulting fees from DePuy Synthes, participates on Data Safety Monitoring Board of SI Bone and the Medical Advisory Board of Injectsense, has a leadership role in the International Society for Hydrocephalus and CSF Disorders, and receives stock or stock options for Injectsense. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study and its components were reviewed and approved by the Mayo Clinic Institutional Review Board (Registration No. IRB00000020). Subject’s participation in the study was entirely voluntary with consent to participate received upon subject’s submission of survey answers. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Glotzbecker MP, Bono CM, Wood KB, et al. Thromboembolic disease in spinal surgery: a systematic review. Spine (Phila Pa 1976) 2009;34:291-303. [Crossref] [PubMed]
- Nemeth B, Lijfering WM, Nelissen RGHH, et al. Risk and Risk Factors Associated With Recurrent Venous Thromboembolism Following Surgery in Patients With History of Venous Thromboembolism. JAMA Netw Open 2019;2:e193690. [Crossref] [PubMed]
- Bono CM, Watters WC 3rd, Heggeness MH, et al. An evidence-based clinical guideline for the use of antithrombotic therapies in spine surgery. Spine J 2009;9:1046-51. [Crossref] [PubMed]
- Burness CB, Perry CM. Rivaroxaban: a review of its use in the treatment of deep vein thrombosis or pulmonary embolism and the prevention of recurrent venous thromboembolism. Drugs 2014;74:243-62. [Crossref] [PubMed]
- Hanley CM, Kowey PR. Are the novel anticoagulants better than warfarin for patients with atrial fibrillation? J Thorac Dis 2015;7:165-71. [PubMed]
- Papadopoulos DV, Kostas-Agnantis I, Gkiatas I, et al. The role of new oral anticoagulants in orthopaedics: an update of recent evidence. Eur J Orthop Surg Traumatol 2017;27:573-82. [Crossref] [PubMed]
- Erdoes G, Martinez Lopez De Arroyabe B, Bolliger D, et al. International consensus statement on the peri-operative management of direct oral anticoagulants in cardiac surgery. Anaesthesia 2018;73:1535-45. [Crossref] [PubMed]
- Leitch J, van Vlymen J. Managing the perioperative patient on direct oral anticoagulants. Can J Anaesth 2017;64:656-72. [Crossref] [PubMed]
- Cuker A, Siegal DM, Crowther MA, et al. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol 2014;64:1128-39. [Crossref] [PubMed]
- Shah SB, Pahade A, Chawla R. Novel reversal agents and laboratory evaluation for direct-acting oral anticoagulants (DOAC): An update. Indian J Anaesth 2019;63:169-81. [Crossref] [PubMed]
- Lassen MR, Gent M, Kakkar AK, et al. The effects of rivaroxaban on the complications of surgery after total hip or knee replacement: results from the RECORD programme. J Bone Joint Surg Br 2012;94:1573-8. [Crossref] [PubMed]
- Mesko JW, Brand RA, Iorio R, et al. Venous thromboembolic disease management patterns in total hip arthroplasty and total knee arthroplasty patients: a survey of the AAHKS membership. J Arthroplasty 2001;16:679-88. [Crossref] [PubMed]
- Du W, Zhao C, Wang J, et al. Comparison of rivaroxaban and parnaparin for preventing venous thromboembolism after lumbar spine surgery. J Orthop Surg Res 2015;10:78. [Crossref] [PubMed]
- Hur M, Park SK, Koo CH, et al. Comparative efficacy and safety of anticoagulants for prevention of venous thromboembolism after hip and knee arthroplasty. Acta Orthop 2017;88:634-41. [Crossref] [PubMed]
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91. [Crossref] [PubMed]
- Sherwood MW, Douketis JD, Patel MR, et al. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Circulation 2014;129:1850-9. [Crossref] [PubMed]
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093-104. [Crossref] [PubMed]
- Bracey A, Shatila W, Wilson J. Bleeding in patients receiving non-vitamin K oral anticoagulants: clinical trial evidence. Ther Adv Cardiovasc Dis 2018;12:361-80. [Crossref] [PubMed]
- Eriksson BI, Borris LC, Dahl OE, et al. A once-daily, oral, direct Factor Xa inhibitor, rivaroxaban (BAY 59-7939), for thromboprophylaxis after total hip replacement. Circulation 2006;114:2374-81. [Crossref] [PubMed]
- Boyd RA, DiCarlo L, Mandema JW. Direct Oral Anticoagulants Vs. Enoxaparin for Prevention of Venous Thromboembolism Following Orthopedic Surgery: A Dose-Response Meta-analysis. Clin Transl Sci 2017;10:260-70. [Crossref] [PubMed]
- Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008;358:2765-75. [Crossref] [PubMed]
- Turpie AG, Lassen MR, Davidson BL, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 2009;373:1673-80. [Crossref] [PubMed]


