
Transforaminal lumbar interbody fusion with a silicon nitride cage demonstrates early radiographic fusion
Introduction
Low back pain is the number one cause of adult disability worldwide (1), with significant implications on healthcare costs and lost employment productivity in the United States (2). Back pain results commonly from degenerative disc disease, spinal stenosis, spondylolisthesis or a combination of these conditions (3,4). As a first line treatment, nonsurgical measures may be effective in relieving back pain, but patients with severe or recalcitrant symptoms may require spinal decompression surgery with segmental fusion (5). Spinal fusion techniques include posterolateral fusion (PLF), a term referring to the fusion of two or more lumbar vertebral bodies by placing bone graft along the sides of the vertebral bodies. This is completed in conjunction with screws and rods to provide immediate stability of the operative levels and encourage bone healing. In transforaminal lumbar interbody fusion (TLIF), pedicle screws and rods are used for spinal fixation, with bone graft placed in the intervertebral disc space and lateral gutters posteriorly (6). Although TLIF involves more surgery, it combines a PLF and interbody fusion which has theoretical advantages outlined below.
While both PLF and TLIF are effective in treating back and radicular pain from degenerative lumbar spinal pathology (6-8), TLIF may have improved radiographic fusion rates when compared to PLF alone (9). Other authors disagree; stating that the addition of an interbody fusion in TLIF does not necessarily improve clinical outcomes (3,8). In theory, the placement of interbody bone graft in TLIF has the advantage of fusing the axial, load bearing axis, with restoration of the disc space assisted by an interbody cage (3,8). Practically, patient pain scores, disability scores, fusion rates and complication rates may not differ between PLF and TLIF (10). It has been suggested that TLIF may be associated with increases in operative time, blood loss and higher costs, although other studies have shown similar readmission rates, pain outcomes and blood loss (11). To summarize, the superiority of TLIF or PLF alone in instrumented lumbar fusion remains unclear.
It is possible that the material properties of the interbody cage may affect fusion rates. One biomaterial, silicon nitride (Si3N4) ceramic has demonstrated to be a safe and effective interbody implant for lumbar spinal fusion (12). Surface bioactivity of Si3N4 has been shown to have enhanced osteoconductive and osteoinductive properties as well as resistance to bacterial adhesion; these material attributes have been well described in several studies (13-18). We hypothesized that TLIF performed with Si3N4 cages may show superior outcomes to PLF, specifically earlier fusion, improved disc and foraminal heights with no increase in complication rates. We present the following article in accordance with the STROBE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-21-115/rc).
Methods
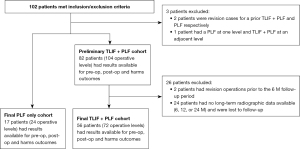
We retrospectively evaluated two cohorts of patients who underwent spinal fusion by a single spine surgeon from August 2013 to January 2017. A total of 99 patients were included (17 PLF and 82 TLIF + PLF). Patient accountability is shown in Figure 1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Parkview Institutional Review Board (No. PRC17-1009 TLIF) on January 31st, 2018 and there was no written consent required for patients due to the retrospective nature of the study. Inclusion criteria included the following: (I) age ≥18 years; (II) lumbar degenerative disease with or without spondylolisthesis; (III) failure of ≥6 weeks of non-surgical treatments; (IV) PLF or TLIF performed 1 or 2 levels. Exclusion criteria included: (I) corpectomy; (II) non-instrumented PLF; (III) history of lumbar trauma, neoplasm, or infection. Patient indications for PLF vs. TLIF were identical, and they were used at the discretion of the primary surgeon. Interbody spacers were made of Si3N4 (SINTX Technologies; Salt Lake City, UT, USA). A standard TLIF technique was performed using shaver blades, curettes, and pituitaries for the discectomy.
Patient data were collected through an electronic medical record system [Centricity Picture Archiving and Communication System (PACS)], and stored on an encrypted server. Data were collected preoperatively, intraoperatively, and at scheduled follow-up visits at 2 weeks, 6 weeks, 3 months, 6 months, 1 year, and 2+ years after surgery. Because of scheduling variability in the patient follow-up windows, time to surgery was also collected for each patient’s return visit. Anterior-posterior, lateral, flexion and extension radiographs were obtained at each follow-up interval for each patient. All radiographic measurements were obtained by the measurement tools in PACS, and stored on a secure server. Radiographs were examined for evidence of: (I) bony bridging; (II) osseous integration of the implant; (III) index surgical level segmental motion; (IV) disc height; (V) foraminal height; (VI) lumbar lordosis (LL); (VII) pelvic incidence (PI); and (VIII) pelvic tilt.
Bony bridging was graded on anterior-posterior and lateral radiographs using the Lenke 5-point modified intertransverse fusion scale, where 1= solid bridging bilaterally, 2= solid bridging unilaterally with questionable bridging contralaterally, 3= questionable bridging bilaterally, 4= questionable bridging unilaterally with no bridging contralaterally, 5= no bridging bilaterally (19). Osseous integration of the Si3N4 implant was graded as “positive” if bridging cancellous bone was visible between fused segments, with no peri-implant radiolucency. For the TLIF patients, fusion was considered to be achieved with either a Lenke score of 1 or 2, or confirmation of osseous integration of the Si3N4 cage.
Index level segmental motion was calculated as the absolute value of the difference on flexion and extension radiographs of the Cobb angle. The Cobb angle was measured, using the measurement tool in PACS, at the intersection of the two lines perpendicular to the two lines drawn along the lower endplate of the superior vertebral body and the upper endplate of the inferior vertebral body. Disc height was measured preoperative and at each post-operative timepoint to assess for implant subsidence. Disc height was measured as the distance between the middle of the bottom surface of the upper vertebra and the middle of the top surface of the lower vertebra. Foraminal height was measured as the maximal diameter of the neural foramen identified on the lateral X-ray view.
LL was measured on lateral radiographs as the angle between the line perpendicular to the superior endplate of S1 and the line perpendicular to the inferior endplate of T12. For the purposes of this text, “lumbar lordosis” and “global lordosis” are equivalent. PI was measured as the angle between two lines: (I) the line perpendicular to the top surface of S1 and (II) the line connecting the center of the femoral head and the midpoint of the top surface of S1. To evaluate restoration of spinal alignment parameters, we calculated the absolute values of the change of LL and of the change of PI, from the preoperative visit to first early follow-up and from the first early to last long-term follow-up. Mismatch between PI and LL (PI-LL mismatch) was calculated as the absolute value of the difference between the PI and LL.
Statistical analysis
Statistical analyses were performed using MedCalc Ver. 20.011-64 bit (Ostend, Belgium). Ordinal data were analyzed using Student’s t-tests whereas nominal results used proportionality assessments including Chi-squared and Fisher’s exact tests. Significance was set at P values of <0.05. Patients who didn’t follow up at a given interval were not included in analysis at that time interval. All 99 patients who met inclusion/exclusion criteria were included at all follow-up intervals that data were available.
Results
One hundred and two patients met inclusion/exclusion criteria; of these, baseline and early post-operative data were available for 99 patients (17 in the PLF group and 82 in the TLIF group) as outlined in Figure 1. Radiographs at 2 weeks, 6 weeks, 3 months and 6 months, 1 year, and >2-year follow-up were available for 56 patient in the TLIF cohort (72 operative levels), and 17 patients in the PLF group (24 operative levels), such that complete long-term follow-up data was available for 73 patients.
Baseline demographic characteristics and comorbidities are shown in Table 1. The PLF group was slightly older (62.4 vs. 54.8 years, P=0.02) with a lower incidence of prior lumbar interventions (5.9% vs. 33.9%, P=0.02) than the TLIF group. The L4/5 fusion level was the most common operative level, with no difference in the distribution of fusion levels between groups. The cohort of patients who had long-term radiographic data had similar distributions of baseline characteristics. Bone graft was commonly utilized intraoperatively with a heterogenous distribution of graft choice between groups. Almost all patients had iliac crest autograft in combination with some form of allograft or bone void filler added to the autograft.
Table 1
| Patient characteristics | PLF | TLIF | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean ± SD or % | N | Mean ± SD or % | ||||||
| Demographics | |||||||||
| Age (years) | 17 | 62.4±10.1 | 56 | 54.8±12 | 0.02 | ||||
| Female (%) | 5 | 29.40 | 26 | 46.40 | 0.22 | ||||
| Average body mass index (kg/m2) | 17 | 32.4±4 | 56 | 31.1±5.1 | 0.34 | ||||
| Comorbidities (%) | |||||||||
| Tobacco use | 5 | 29.40 | 17 | 30.40 | 0.94 | ||||
| Diabetes mellitus | 5 | 29.40 | 11 | 19.60 | 0.40 | ||||
| Osteoporosis | 1 | 5.90 | 1 | 1.80 | 0.37 | ||||
| Osteopenia | 0 | 0.00 | 3 | 5.40 | 0.33 | ||||
| Hypertension | 13 | 76.50 | 33 | 58.90 | 0.19 | ||||
| Other comorbidities | 4 | 23.50 | 18 | 32.10 | 0.50 | ||||
| Prior lumbar interventions | 1 | 5.90 | 19 | 33.90 | 0.02 | ||||
| Pre-operative radiographic parameters | |||||||||
| Segmental lordosis (°) | 23 | 17.65±10.05 | 71 | 20.04±7.44 | 0.22 | ||||
| Global lordosis (°) | 16 | 47.44±10.63 | 55 | 54.76±13.41 | 0.049 | ||||
| Pelvic incidence (°) | 15 | 53.53±9.17 | 52 | 52.65±12.01 | 0.79 | ||||
| PI-LL mismatch | 15 | 10.27±7.2 | 52 | 7.96±6.1 | 0.22 | ||||
| Pelvic tilt (°) | 15 | 23.73±7.77 | 51 | 18.76±6.94 | 0.02 | ||||
| Disc height (mm) | 24 | 6.67±4.58 | 71 | 7.73±3.04 | 0.2 | ||||
| Foraminal height (mm) | 24 | 14.58±5.09 | 71 | 13.45±5.44 | 0.37 | ||||
| Operative details (%) | |||||||||
| 1-level fusion | 10 | 41.70 | 40 | 55.60 | 0.24 | ||||
| 2-level fusion | 14 | 58.30 | 32 | 44.40 | 0.24 | ||||
| L1/L2 fusion level | 1 | 4.20 | 0 | 0.00 | 0.08 | ||||
| L2/L3 fusion level | 1 | 4.20 | 1 | 1.40 | 0.41 | ||||
| L3/L4 fusion level | 5 | 20.80 | 8 | 11.10 | 0.23 | ||||
| L4/L5 fusion level | 12 | 50.00 | 33 | 45.80 | 0.72 | ||||
| L5/S1 fusion level | 5 | 20.80 | 30 | 41.70 | 0.07 | ||||
PLF, posterolateral fusion; TLIF, transforaminal lumbar interbody fusion; PI, pelvic incidence; LL, lumbar lordosis.
Radiographic outcomes
Tables 2,3 show radiographic outcomes for the study groups, including average follow-up duration for patients with long-term follow up. There were no differences in the pre-op disc height between groups (6.67 mm PLF vs. 7.73 mm, P=0.20). At every post-operative interval there was a significant improvement in disc height in the TLIF group compared to PLF alone. The change in disc height between pre-operative radiographs and each follow-up timepoint was also compared between groups Figure 2. The TLIF group experienced on average 4 mm gain in disc height postoperatively that was maintained over all follow-up intervals.
Table 2
| Patient characteristics | PLF | TLIF | P value | |||
|---|---|---|---|---|---|---|
| N | Mean ± SD or % | N | Mean ± SD or % | |||
| 2-week follow up | ||||||
| Average follow up (weeks) | 24 | 1.92±0.44 | 72 | 2.02±0.43 | 0.338 | |
| Segmental lordosis (°) | 24 | 15.96±9.27 | 72 | 22.85±5.39 | 0 | |
| Global lordosis (°) | 17 | 42.47±12.08 | 56 | 54.86±10.98 | 0 | |
| Pelvic incidence (°) | 12 | 56.00±8.26 | 46 | 53.24±10.73 | 0.412 | |
| PI-LL mismatch | 12 | 14.50±16.18 | 45 | 7.49±5.67 | 0.018 | |
| Pelvic tilt (°) | 12 | 27.83±7.17 | 46 | 21.02±6.62 | 0.028 | |
| Disc height (mm) | 24 | 7.79±4.79 | 72 | 12.42±2.24 | 0 | |
| ∆ in disc height from pre-op (mm) | 24 | 1.13±2.19 | 71 | 4.66±2.74 | 0 | |
| Foraminal height (mm) | 24 | 14.58±4.67 | 72 | 16.47±3.70 | 0.046 | |
| ∆ in foraminal height (mm) | 24 | 0±2.99 | 71 | 3.00±4.01 | 0.001 | |
| 3-month follow up | ||||||
| Average follow up (weeks) | 24 | 12.48±1.61 | 65 | 12.18±1.33 | 0.389 | |
| Segmental lordosis (°) | 24 | 18.29±9.32 | 65 | 22.75±4.87 | 0.004 | |
| Global lordosis (°) | 17 | 47.53±11.07 | 51 | 56.69±10.51 | 0.003 | |
| Pelvic incidence (°) | 16 | 53.94±9.31 | 36 | 51.14±11.24 | 0.388 | |
| PI-LL mismatch | 16 | 11.13±11.26 | 36 | 7.25±4.16 | 0.074 | |
| Pelvic tilt (°) | 16 | 24.56±6.47 | 36 | 19.83±5.88 | 0.012 | |
| Disc height (mm) | 24 | 7.92±4.79 | 65 | 12.32±2.40 | 0 | |
| ∆ in disc height from pre-op (mm) | 24 | 1.25±2.07 | 64 | 4.52±3.01 | 0 | |
| Foraminal height (mm) | 24 | 13.92±4.33 | 65 | 16.37±3.90 | 0.012 | |
| ∆ in foraminal height (mm) | 24 | −0.67±3.50 | 64 | 3.05±3.63 | 0 | |
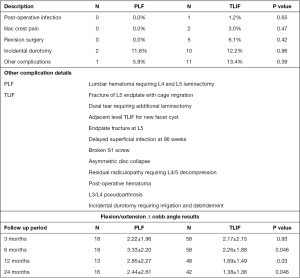
| ∆ cobb angle (°) | 18 | 2.22±1.96 | 58 | 2.17±2.15 | 0.930 | |
| Intertransverse fusion grade [1–5] | 24 | 4.46±0.88 | 64 | 4.38±1.03 | 0.737 | |
| Osseous integration (1= yes; 2= no) | NA | NA | 65 | 1.62±0.49 | NA | |
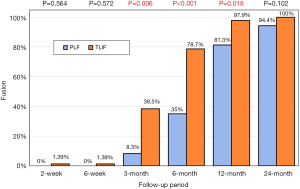
| Fusion (%) | 24 | 8.30 | 64 | 38.50 | 0.006 | |
∆, change in. PLF, posterolateral fusion; TLIF, transforaminal lumbar interbody fusion; PI, pelvic incidence; LL, lumbar lordosis.
Table 3
| Patient characteristics | PLF | TLIF | P value | ||||
|---|---|---|---|---|---|---|---|
| N | Mean ± SD or % | N | Mean ± SD or % | ||||
| 6-month follow up | |||||||
| Average follow up (weeks) | 20 | 24.99±2.81 | 61 | 25.19±1.98 | 0.731 | ||
| Segmental lordosis (°) | 20 | 16.10±10.24 | 58 | 21.98±5.43 | 0.002 | ||
| Global lordosis (°) | 14 | 46.29±11.39 | 45 | 56.71±11.00 | 0.003 | ||
| Pelvic incidence (°) | 12 | 55.25±8.50 | 40 | 51.58±10.02 | 0.256 | ||
| PI-LL mismatch | 12 | 10.25±11.27 | 40 | 7.80±4.98 | 0.284 | ||
| Pelvic tilt (°) | 12 | 23.83±7.80 | 40 | 19.38±6.31 | 0.048 | ||
| Disc height (mm) | 20 | 7.35±4.50 | 61 | 12.11±2.30 | 0 | ||
| ∆ in disc height from pre-op (mm) | 20 | 0.80±2.33 | 60 | 4.37±3.05 | 0 | ||
| Foraminal height (mm) | 20 | 14.55±3.97 | 61 | 16.23±4.06 | 0.110 | ||
| ∆ in foraminal height (mm) | 20 | −0.05±3.75 | 60 | 2.53±4.30 | 0.019 | ||
| ∆ cobb angle (°) | 18 | 3.33±2.20 | 58 | 2.26±1.88 | 0.046 | ||
| Intertransverse fusion grade [1–5] | 20 | 2.95±1.36 | 61 | 2.92±1.36 | 0.932 | ||
| Osseous integration (1= yes; 2= no) | NA | NA | 61 | 1.21±0.41 | NA | ||
| Fusion (%) | 20 | 35.00 | 61 | 78.70 | 0 | ||
| 12-month follow up | |||||||
| Average follow up (weeks) | 16 | 55.54±9.73 | 48 | 56.57±11.43 | 0.758 | ||
| Segmental lordosis (°) | 15 | 15.47±10.13 | 48 | 21.10±6.00 | 0.010 | ||
| Global lordosis (°) | 11 | 48.55±13.42 | 38 | 59.18±10.46 | 0.008 | ||
| Pelvic incidence (°) | 11 | 54.55±6.71 | 31 | 52.94±11.25 | 0.659 | ||
| PI-LL mismatch | 10 | 12.90±13.92 | 31 | 9.29±4.88 | 0.219 | ||
| Pelvic tilt (°) | 11 | 23.45±8.17 | 31 | 18.84±5.71 | 0.047 | ||
| Disc height (mm) | 16 | 6.75±4.88 | 48 | 11.75±2.28 | 0 | ||
| ∆ in disc height from pre-op (mm) | 16 | 1.25±1.88 | 47 | 4.09±3.11 | 0.001 | ||
| Foraminal height (mm) | 16 | 13.88±4.47 | 48 | 15.71±3.45 | 0.094 | ||
| ∆ in foraminal height (mm) | 16 | −0.63±1.96 | 47 | 1.87±4.08 | 0.022 | ||
| ∆ cobb angle (°) | 13 | 2.85±2.27 | 48 | 1.69±1.49 | 0.031 | ||
| Intertransverse fusion grade [1–5] | 16 | 1.81±1.11 | 48 | 1.56±0.85 | 0.350 | ||
| Osseous integration (1= yes; 2= no) | NA | NA | 48 | 1.02±0.14 | NA | ||
| Fusion (%) | 16 | 81.30 | 48 | 97.90 | 0.018 | ||
| 24-month follow up | |||||||
| Average follow up (weeks) | 18 | 182.37±172.61 | 48 | 136.66±34.55 | 0.083 | ||
| Segmental lordosis (°) | 18 | 16.94±8.30 | 48 | 21.85±5.82 | 0.009 | ||
| Global lordosis (°) | 12 | 43.42±12.12 | 35 | 57.86±8.93 | 0 | ||
| Pelvic incidence (°) | 11 | 55.18±10.97 | 33 | 53.61±7.92 | 0.609 | ||
| PI-LL mismatch | 11 | 13.45±13.49 | 33 | 8.64±5.11 | 0.090 | ||
| Pelvic tilt (°) | 11 | 25.91±11.07 | 33 | 20.12±6.05 | 0.033 | ||
| Disc height (mm) | 18 | 7.78±5.68 | 48 | 12.31±1.89 | 0 | ||
| ∆ in disc height from pre-op (mm) | 18 | 1.50±3.65 | 48 | 4.38±2.74 | 0.001 | ||
| Foraminal height (mm) | 18 | 14.33±4.37 | 48 | 15.85±3.07 | 0.117 | ||
| ∆ in foraminal height (mm) | 18 | 0.44±4.73 | 48 | 1.35±3.82 | 0.423 | ||
| ∆ cobb angle (°) | 16 | 2.44±2.61 | 42 | 1.38±1.36 | 0.048 | ||
| Intertransverse fusion grade [1–5] | 18 | 1.33±0.77 | 48 | 1.31±0.75 | 0.924 | ||
| Osseous integration (1= yes; 2= no) | NA | NA | 48 | 1.00±0 | NA | ||
| Fusion (%) | 18 | 94.40 | 48 | 100.00 | 0.102 | ||
∆, change in. PLF, posterolateral fusion; TLIF, transforaminal lumbar interbody fusion; PI, pelvic incidence; LL, lumbar lordosis.
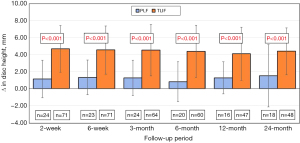
There were no pre-operative differences in foraminal height between study groups (14.58 mm PLF vs. 13.45 mm TLIF, P=0.37). The TLIF group showed a significant improvement in average foraminal height of ~3 mm at 2 weeks, 6 weeks and 3 months compared to PLF. However, at 6, 12, and 24 months of follow-up, this difference resolved such that foraminal height was similar between study groups.
There was no statistically significant difference in intertransverse fusion scores at any timepoint between TLIF and PLF. The mean intertransverse fusion score was less than 2 at the 12-month interval, indicating radiographic fusion, on average, by 12 months in either group. Composite fusion scores (which added the finding of osseous integration of the interbody cage to intertransverse fusion scores) are shown in Figure 3. Composite fusion scores showed improved fusion in the TLIF vs. PLF group at 3, 6 and 12 months; this difference resolved itself at the 24-month follow up, when >90% of PLF patients and 100% TLIF patients had achieved spinal fusion.
Index level segmental motion data was available at 3 months, and at the subsequent follow up intervals. Comparing Cobb angles derived from flexion and extension radiographs (Figure 4), TLIF patients had significantly less segmental motion than PLF patients at 6-months, and subsequent follow up intervals. On average, the TLIF group had one degree less segmental motion at all long term follow up points, with an average <2 degrees segmental motion at the 12- and 24-month intervals.
Global and segmental lordosis
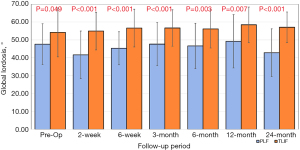
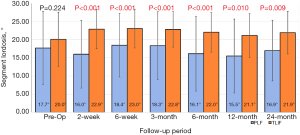
The two groups differed pre-operatively with respect to global lordosis, with TLIF patients having greater global lordosis compared to PLF (54.76° vs. 47.44°, P=0.049). There was no decrease in global lordosis for the TLIF patients after surgery, while the PLF group was more variable, with some patients showing a loss of 5 degrees while others a gain of 2 degrees in lordosis at various follow up intervals (Figure 5). Pre-operatively there was no difference in segmental lordosis between TLIF and the PLF alone groups (20.04° vs. 17.65°, P=0.224). At every additional follow-up interval there was a significantly greater segmental lordosis observed in the TLIF group compared to the PLF group (Figure 6).
Spinal alignment
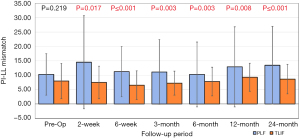
PI and pelvic tilt were measured pre-operatively, and at each follow-up visit. There was no difference in PI between TLIF and PLF groups pre-operatively or at any time point after surgery. Pelvic tilt was consistently less in the TLIF group compared to the PLF alone group across all follow up intervals. At 24 months there was >5 degrees difference between groups with an average pelvic tilt (PT) of 25.9° and 20.1° for TLIF vs. PLF respectively. There was no observed preoperative difference in PI-LL mismatch, but greater mismatch rates in the PLF group at both early and late follow up timepoints (Figure 7).
Complications
Perioperative complications were compared between groups at outlined in Figure 4. Dural tears were the most common complication in this series, with 10 patients in the TLIF group, and 2 in the PLF group (TLIF 12.2%, PLF 11.8%, P=0.96). Other complications similarly did not differ between groups. Five of the 82 TLIF patients required hardware revision; none among the PLF group. Two revision operations were done because of vertebral body endplate fracture and implant subsidence, one revision operation for persistent radiculopathy at the operated level, one revision operation for a broken S1 screw and one revision for pseudoarthrosis at 8 months which progressed to fusion at the 24-month follow up visit. One hematoma in the PLF group resolved after similar treatment.
Discussion
The aim of this retrospective study was to compare fusion rates, relevant radiographic variables, and complication rates for patients undergoing PLF versus TLIF; the latter patients had a Si3N4 cage implanted for the intervertebral component of the surgery. Most studies comparing PLF to TLIF leave the choice of fusion technique to surgeon discretion and judgment; as such, there is limited evidence to support one operation over another (20,21). Høy et al. randomized patients to TLIF with a tantalum cage vs. PLF with a follow-up interval of 2 years and found no difference in SF-36 or DPQ pain scores, length of stay or complication rate (7). A recent non-inferiority randomized controlled-trial by Kersten et al. compared TLIF with a Si3N4 cage to utilization of a PEEK cage. They reported no difference in RMDQ, SF-36 or ODI scores, complication rates as well as no differences in radiographic fusion between groups (22).
Similar to other studies, we relied on plain radiographs of operative levels, in combination with clinical findings to assess lumbar fusion (23). When compared to other imaging modalities such as fine-cut computed tomography, plain X-rays are advantageous in terms of lower cost, ease of use, lower radiation exposure, and the documented accuracy and reproducibility of plain radiographs in this regard (24-27).
Our data showed earlier radiographic fusion at 3, 6 and 12 months of TLIF over PLF, this advantage persisted even at 24 months even though the difference was not statistically significant (100% TLIF, 94.4% PLF). Studies have shown the influence of modifiable and non-modifiable risk factors related to bone quality and healing (diabetes, smoking, obesity, osteoporosis, female sex, age) as well as bone graft utilization in affecting fusion and reoperation rates (28-32); these factors were comparable between study groups, except for greater patient age in the PLF group. There is a well understood age related decline in bone mineral density, and despite age differences between our groups there was no appreciable difference in osteopenia/osteoporosis rates.
With the axial support of a structural interbody cage in TLIF, it is not surprising that we found greater disc height restoration (4 mm average) maintained through the 2-year follow-up point in the TLIF group versus the PLF group. Foraminal height was significantly greater in the TLIF group, but this difference resolved itself at 6, 12, or 24 months between study groups. Tallarico et al. compared mechanical loading of normal harvested cadaveric spine specimens to specimens following a TLIF procedure. This biomechanical study demonstrated that TLIF specimens had a 1.5–2 mm gain in neuroforaminal height, with elimination of foraminal stenosis on flexion and extension compared to non-surgical spine specimens. Additionally, they noted that the greatest decompression was achieved with posterior positioning of the interbody cage compared to anterior cage placement (33). While the present study did not measure radicular leg pain, foraminal height is an accepted surrogate for nerve root decompression and corresponding pain relief (34). Likewise, disc height is correlated with the restoration of lumbar lordosis and overall sagittal balance (35).
In the present study, the TLIF patients had greater construct rigidity with an average of 1° less segmental motion on flexion-extension radiographs at each follow-up interval (Figure 4). Previous reports have used Cobb angle measurements on flexion-extension radiographs, stating that a difference of <2° to suggest fusion (36-38), with >4° difference associated with pseudoarthrosis (39,40). Our TLIF group showed <2° of segmental motion at both 12 and 24 months, indicating spinal stability and successful fusion.
Sagittal balance is a key factor in understanding the development of degenerative spinal pathology, and improvement in this variable may reduce the risk of adjacent segment disease after spinal fusion (41). In the present study, segmental and global lordosis values were improved at all follow-up time periods for the TLIF group while the PLF group had more variable segmental and global lordosis. Additionally, the TLIF group demonstrated lower pelvic tilt measurements compared to PLF patients. Increased pelvic tilt has been identified as an independent risk factor for post-operative pain following lumbosacral fusion (42-44). Lazennec et al. followed lumbosacral fusion patients (mean follow-up 2.8 years) and identified patients without post fusion pain had a PT of 13.9° compared to those with pain had a PT of 26.2° (42). As outlined in Table 3, our results demonstrated at 24-month the PT of the two groups differed by >5 degrees, with an average PT of 25.91 in the PLF group and 20.12 in the TLIF group (P=0.033).
Instrumented surgical fusion for degenerative spinal pathology is not without risk. Previous reports of TLIF and PLF surgery have shown that complications such as nerve injury, dural tears, implant or bone graft migration, infection, implant subsidence and failure of fusion can manifest (45). A metanalysis of 990 patients undergoing TLIF or PLF reported a >50% overall lower complication rate following TLIF compared to PLF (46). In our study population, five of 82 TLIF patients required revision surgery after the index procedure, and two experienced implant subsidence. No patients in the PLF group required further hardware revision. While silicon nitride is stiffer than both titanium and PEEK, the material modulus is unrelated to the risk of implant subsidence (47). No clinical studies have demonstrated higher rates of subsidence as well. Si3N4 cages offer specific benefits in terms of ease of radiographic imaging, as well as accelerated bone healing and resistance to bacterial infection (48). These advantages have been validated in a number of in vitro as well as large scale clinical studies (49,50).
Given the small patient population outlined in our study, multicenter registries and metanalyses may offer more accurate insights into complication rates given their larger sample populations. A recent metanalysis by Levin et al. concluded there was no difference between TLIF and PLF patients for post-operative infection rate (3.3% vs. 3.4%, P=0.90) or other complications such as length of stay, readmission rate or rate of durotomy (51). Zhang et al. completed a similar metanalysis for degenerative lumbar spondylosis and found no increase in reoperation rate for TLIF vs. PLF [relative risk (RR) =0.83, P=0.809] or increase in overall complication rate (RR =1.72, P=0.166) (10).
Our results were limited both by sample size and inconsistent patient follow-up intervals. In addition to limited patient numbers and attendant reduction in statistical power, the non-randomized retrospective nature of this study is another a limitation of the present report. Baseline foraminal heights also differed pre-operatively between the two groups, thereby limiting out ability to conclude that foraminal height restoration was directly related to the TLIF procedure itself. Although Cobb angle measurements are a validated metric (52), radiographic evaluations were completed by one surgeon, and not someone blinded to the study. No patient-reported outcomes or pain scales were collected; although these outcomes are extensively discussed in the literature (53). Finally we were not able to control for the bone graft utilization between cohorts, but it remained relatively consistent for the surgeon throughout the study interval.
Conclusions
Our data suggest superiority of TLIF over PLF, in terms of radiographically-adjudicated fusion rates, disc and foraminal height restoration and increased segmental rigidity, without an increase in complication rates. All TLIF patients had successful radiographic fusion at 24 months, with the PLF patients achieving almost the same success. While not proven in this study, the enhanced osteogenic properties of Si3N4 interbody cases may have contributed to the observed differences between TLIF over PLF. Further studies are warranted to explore this hypothesis.
Acknowledgments
The authors thank Lauren Jagger for her assistance in early data collection. The authors would like to thank Michael Hanna, PhD, for his assistance in reviewing the results.
Funding: This study was funded by the Student Research Fellowship Program of the Indiana University School of Medicine in Fort Wayne, Indiana. This work was additionally supported in part by Parkview Research Center and the Mirro Center for Research and Innovation in Fort Wayne, Indiana.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-21-115/rc
Data Sharing Statement: Available at https://jss.amegroups.com/article/view/10.21037/jss-21-115/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-21-115/coif). BSB serves as an unpaid editorial board member of from Journal of Spine Surgery from March 2020 to February 2022, and is an employee and stockholder of STINX Corporation (formerly Amedica Corporation) which manufactured the silicon nitride devices used in this study. MWS was a consulting surgeon of Amedica Corporation. BM was an employee and stockholder, now retired on Dec. 31, 2021, but retained as a consultant at STINX Corporation. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Parkview Institutional Review Board (No. PRC17-1009 TLIF) on January 31st, 2018 and there was no written consent required for patients due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:743-800. [Crossref] [PubMed]
- Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. Lancet 2018;391:2356-67. [Crossref] [PubMed]
- Lee YC, Zotti MG, Osti OL. Operative management of lumbar degenerative disc disease. Asian Spine J 2016;10:801-19. [Crossref] [PubMed]
- Jalalpour K, Neumann P, Johansson C, et al. A randomized controlled trial comparing transforaminal lumbar interbody fusion and uninstrumented posterolateral fusion in the degenerative lumbar spine. Global Spine J 2015;5:322-8. [Crossref] [PubMed]
- Matz PG, Meagher RJ, Lamer T, et al. Guideline summary review: an evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. Spine J 2016;16:439-48. [Crossref] [PubMed]
- Lee N, Kim KN, Yi S, et al. Comparison of outcomes of anterior, posterior, and transforaminal lumbar interbody fusion surgery at a single lumbar level with degenerative spinal disease. World Neurosurg 2017;101:216-26. [Crossref] [PubMed]
- Høy K, Bünger C, Niederman B, et al. Transforaminal lumbar interbody fusion (TLIF) versus posterolateral instrumented fusion (PLF) in degenerative lumbar disorders: a randomized clinical trial with 2-year follow-up. Eur Spine J 2013;22:2022-9. [Crossref] [PubMed]
- Tropiano P, Giorgi H, Faure A, et al. Surgical techniques for lumbo-sacral fusion. Orthop Traumatol Surg Res 2017;103:S151-9. [Crossref] [PubMed]
- Cole CD, McCall TD, Schmidt MH, et al. Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med 2009;2:118-26. [Crossref] [PubMed]
- Zhang BF, Ge CY, Zheng BL, et al. Transforaminal lumbar interbody fusion versus posterolateral fusion in degenerative lumbar spondylosis: A meta-analysis. Medicine (Baltimore) 2016;95:e4995. [Crossref] [PubMed]
- Glassman SD, Carreon LY, Ghogawala Z, et al. Benefit of transforaminal lumbar interbody fusion vs posterolateral spinal fusion in lumbar spine disorders: a propensity-matched analysis from the national neurosurgical quality and outcomes database registry. Neurosurgery 2016;79:397-405. [Crossref] [PubMed]
- McEntire BJ, Maslin G, Bal BS. Two-year results of a double-blind multicenter randomized controlled non-inferiority trial of polyetheretherketone (PEEK) versus silicon nitride spinal fusion cages in patients with symptomatic degenerative lumbar disc disorders. J Spine Surg 2020;6:523-40. [Crossref] [PubMed]
- Gorth DJ, Puckett S, Ercan B, et al. Decreased bacteria activity on Si3N4 surfaces compared with PEEK or titanium. Int J Nanomedicine 2012;7:4829-40. [PubMed]
- Bock RM, Jones EN, Ray DA, et al. Bacteriostatic behavior of surface modulated silicon nitride in comparison to polyetheretherketone and titanium. J Biomed Mater Res A 2017;105:1521-34. [Crossref] [PubMed]
- Pezzotti G, Marin E, Adachi T, et al. Bioactive silicon nitride: a new therapeutic material for osteoarthropathy. Sci Rep 2017;7:44848. [Crossref] [PubMed]
- Pezzotti G, Oba N, Zhu W, et al. Human osteoblasts grow transitional Si/N apatite in quickly osteointegrated Si3N4 cervical insert. Acta Biomater 2017;64:411-20. [Crossref] [PubMed]
- Ishikawa M, de Mesy Bentley KL, McEntire BJ, et al. Surface topography of silicon nitride affects antimicrobial and osseointegrative properties of tibial implants in a murine model. J Biomed Mater Res A 2017;105:3413-21. [Crossref] [PubMed]
- Webster TJ, Patel AA, Rahaman MN, et al. Anti-infective and osteointegration properties of silicon nitride, poly(ether ether ketone), and titanium implants. Acta Biomater 2012;8:4447-54. [Crossref] [PubMed]
- Lenke LG, Bridwell KH, Bullis D, et al. Results of in situ fusion for isthmic spondylolisthesis. J Spinal Disord 1992;5:433-42. [Crossref] [PubMed]
- Resnick DK, Watters WC 3rd, Sharan A, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 9: lumbar fusion for stenosis with spondylolisthesis. J Neurosurg Spine 2014;21:54-61. [Crossref] [PubMed]
- Watters WC 3rd, Bono CM, Gilbert TJ, et al. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. Spine J 2009;9:609-14. [Crossref] [PubMed]
- Kersten RFMR, Öner FC, Arts MP, et al. The SNAP trial: 2-year results of a double-blind multicenter randomized controlled trial of a silicon nitride versus a peek cage in patients after lumbar fusion surgery. Global Spine J 2021; Epub ahead of print. [Crossref] [PubMed]
- McAfee PC, Boden SD, Brantigan JW, et al. Symposium: a critical discrepancy-a criteria of successful arthrodesis following interbody spinal fusions. Spine (Phila Pa 1976) 2001;26:320-34. [Crossref] [PubMed]
- Carreon LY, Djurasovic M, Glassman SD, et al. Diagnostic accuracy and reliability of fine-cut CT scans with reconstructions to determine the status of an instrumented posterolateral fusion with surgical exploration as reference standard. Spine (Phila Pa 1976) 2007;32:892-5. [Crossref] [PubMed]
- Fogel GR, Toohey JS, Neidre A, et al. Fusion assessment of posterior lumbar interbody fusion using radiolucent cages: X-ray films and helical computed tomography scans compared with surgical exploration of fusion. Spine J 2008;8:570-7. [Crossref] [PubMed]
- Biswas D, Bible JE, Bohan M, et al. Radiation exposure from musculoskeletal computerized tomographic scans. J Bone Joint Surg Am 2009;91:1882-9. [Crossref] [PubMed]
- Blumenthal SL, Gill K. Can lumbar spine radiographs accurately determine fusion in postoperative patients? Correlation of routine radiographs with a second surgical look at lumbar fusions. Spine (Phila Pa 1976) 1993;18:1186-9. [Crossref] [PubMed]
- Sato S, Yagi M, Machida M, et al. Reoperation rate and risk factors of elective spinal surgery for degenerative spondylolisthesis: minimum 5-year follow-up. Spine J 2015;15:1536-44. [Crossref] [PubMed]
- Konomi T, Yasuda A, Fujiyoshi K, et al. Incidences and risk factors for postoperative non-union after posterior lumbar interbody fusion with closed-box titanium spacers. Asian Spine J 2020;14:106-12. [Crossref] [PubMed]
- vonderHoeh NH, Voelker A, Heyde CE. Results of lumbar spondylodeses using different bone grafting materials after transforaminal lumbar interbody fusion (TLIF). Eur Spine J 2017;26:2835-42. [Crossref] [PubMed]
- Buser Z, Brodke DS, Youssef JA, et al. Synthetic bone graft versus autograft or allograft for spinal fusion: a systematic review. J Neurosurg Spine 2016;25:509-16. [Crossref] [PubMed]
- Wang T, Ding W. Risk factors for adjacent segment degeneration after posterior lumbar fusion surgery in treatment for degenerative lumbar disorders: a meta-analysis. J Orthop Surg Res 2020;15:582. [Crossref] [PubMed]
- Tallarico RA, Lavelle WF, J, Bianco A, et al. Positional effects of transforaminal interbody spacer placement at the L5-S1 intervertebral disc space: a biomechanical study. Spine J 2014;14:3018-24. [Crossref] [PubMed]
- Tian H, Wu A, Guo M, et al. Adequate restoration of disc height and segmental lordosis by lumbar interbody fusion decreases adjacent segment degeneration. World Neurosurg 2018;118:e856-64. [Crossref] [PubMed]
- Feng Y, Chen L, Gu Y, et al. Influence of the posterior lumbar interbody fusion on the sagittal spino-pelvic parameters in isthmic L5-S1 spondylolisthesis. J Spinal Disord Tech 2014;27:E20-5. [Crossref] [PubMed]
- Simmons JW, Andersson GB, Russell GS, et al. A prospective study of 342 patients using transpedicular fixation instrumentation for lumbosacral spine arthrodesis. J Spinal Disord 1998;11:367-74. [Crossref] [PubMed]
- Brodsky AE, Kovalsky ES, Khalil MA. Correlation of radiologic assessment of lumbar spine fusions with surgical exploration. Spine (Phila Pa 1976) 1991;16:S261-5. [Crossref] [PubMed]
- Zdeblick TA. A prospective, randomized study of lumbar fusion. Preliminary results. Spine (Phila Pa 1976) 1993;18:983-91. [Crossref] [PubMed]
- Cannada LK, Scherping SC, Yoo JU, et al. Pseudoarthrosis of the cervical spine: a comparison of radiographic diagnostic measures. Spine (Phila Pa 1976) 2003;28:46-51. [Crossref] [PubMed]
- Lehmann TR, LaRocca HS. Repeat lumbar surgery. A review of patients with failure from previous lumbar surgery treated by spinal canal exploration and lumbar spinal fusion. Spine (Phila Pa 1976) 1981;6:615-9. [Crossref] [PubMed]
- Menezes-Reis R, Bonugli GP, Dalto VF, et al. Association between lumbar spine sagittal alignment and L4-L5 disc degeneration among asymptomatic young adults. Spine (Phila Pa 1976) 2016;41:E1081-7. [Crossref] [PubMed]
- Lazennec JY, Ramaré S, Arafati N, et al. Sagittal alignment in lumbosacral fusion: relations between radiological parameters and pain. Eur Spine J 2000;9:47-55. [Crossref] [PubMed]
- Lafage V, Schwab F, Patel A, et al. Pelvic tilt and truncal inclination: two key radiographic parameters in the setting of adults with spinal deformity. Spine (Phila Pa 1976) 2009;34:E599-606. [Crossref] [PubMed]
- Ould-Slimane M, Lenoir T, Dauzac C, et al. Influence of transforaminal lumbar interbody fusion procedures on spinal and pelvic parameters of sagittal balance. Eur Spine J 2012;21:1200-6. [Crossref] [PubMed]
- Chrastil J, Patel AA. Complications associated with posterior and transforaminal lumbar interbody fusion. J Am Acad Orthop Surg 2012;20:283-91. [Crossref] [PubMed]
- de Kunder SL, van Kuijk SMJ, Rijkers K, et al. Transforaminal lumbar interbody fusion (TLIF) versus posterior lumbar interbody fusion (PLIF) in lumbar spondylolisthesis: a systematic review and meta-analysis. Spine J 2017;17:1712-21. [Crossref] [PubMed]
- Suh PB, Puttlitz C, Lewis C, et al. The effect of cervical interbody cage morphology, material composition, and substrate density on cage subsidence. J Am Acad Orthop Surg 2017;25:160-8. [Crossref] [PubMed]
- Fiani B, Jarrah R, Shields J, et al. Enhanced biomaterials: systematic review of alternatives to supplement spine fusion including silicon nitride, bioactive glass, amino peptide bone graft, and tantalum. Neurosurg Focus 2021;50:E10. [Crossref] [PubMed]
- Calvert GC, Huffmon GV 3rd, Rambo WM Jr, et al. Clinical outcomes for anterior cervical discectomy and fusion with silicon nitride spine cages: a multicenter study. J Spine Surg 2019;5:504-19. [Crossref] [PubMed]
- Calvert GC, VanBuren Huffmon G 3rd, Rambo WM Jr, et al. Clinical outcomes for lumbar fusion using silicon nitride versus other biomaterials. J Spine Surg 2020;6:33-48. [Crossref] [PubMed]
- Levin JM, Tanenbaum JE, Steinmetz MP, et al. Posterolateral fusion (PLF) versus transforaminal lumbar interbody fusion (TLIF) for spondylolisthesis: a systematic review and meta-analysis. Spine J 2018;18:1088-98. [Crossref] [PubMed]
- Morrissy RT, Goldsmith GS, Hall EC, et al. Measurement of the Cobb angle on radiographs of patients who have scoliosis. Evaluation of intrinsic error. J Bone Joint Surg Am 1990;72:320-7. [Crossref] [PubMed]
- Kim E, Chotai S, Stonko D, et al. A retrospective review comparing two-year patient-reported outcomes, costs, and healthcare resource utilization for TLIF vs. PLF for single-level degenerative spondylolisthesis. Eur Spine J 2018;27:661-9. [Crossref] [PubMed]


