
Treatment of thoracolumbar burst fractures: SpineJack vs. posterior arthrodesis—comparison of clinical and radiological outcomes
Introduction
The management of type A2 split and A3 and A4 thoracolumbar burst fractures is still debated. Recently, surgery has been increasingly preferred to conservative treatment in cases without neurological impairments (1) to avoid inactivity and diminution of daily quality of life (2).
Often, thoracolumbar fractures may require anterior column support and correction of the post-traumatic deformity (1,3). Indeed, stabilization of the anterior column remains crucial to avoid correction and instrumentation failure, but it is at the cost of a more invasive approach associated with increased morbidity (1,3-6). Although posterior screw rod arthrodesis is the ‘gold standard’, it restores stability at the cost of paravertebral muscle damage and limited mobility by sacrificing at least two motion segments (1,3). To avoid such an unfavorable biomechanical situation, some authors have proposed to use intrasomatic expandable implants for direct fracture reduction (5,7,8). Of such devices, SpineJack® (SJ, Stryker Corp, Kalamazoo, MI) had overall favorable results in cadaveric and clinical trials, demonstrating satisfactory outcomes in terms of pain relief, midline vertebral height restoration, and low rates of adjacent fractures over time (7).
SpineJack is particularly suitable for treating selected cases of type A2, A3, and A4 vertebral fractures with limited fragment displacement and impairment of the anterior column. The intravertebral implant allows endplate anatomical restoration and kyphosis angle reduction, avoiding late kyphosis and enhancing clinical outcomes by decreasing postoperative pain (7). This paper aims to compare the clinical and radiological outcomes of a sample of patients with A2 split and A3, A4 burst fractures treated either with posterior arthrodesis or SpineJack. We present the following article in accordance with the STROBE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-21-118/rc).
Methods
Patients identification
We prospectively collected and retrospectively analyzed patients diagnosed with types A2, A3, and A4 thoracolumbar split or burst fractures operated on at our institution (Aulss2, Marca Trevigiana, Treviso, Italy) between January 2017 and July 2021. The study was conducted in accordance with the Declaration of Helsinki (revised in 2013). All the data from patients are digitalized and prospectively collected, in an anonymized database for exclusive medical use. This study is a prospective collection and retrospective analysis of outcomes, ethical approval was waived by “Aulss 2 Marca Trevigiana” internal review board of our Institution. Informed consent was taken and stored from all participants to use medical imaging. We examined the records of 196 consecutive patients with a thoracolumbar split or burst fracture comprised between the T11 and L3 levels. We further stratified our sample of A2, A3, and A4 fracture patients based on treatment: posterior arthrodesis with pedicle screws (PA group) and SpineJack implantation (SJ group). Both males and females were included if they met the following inclusion criteria: (I) age ≥18 years; (II) diagnosis of T11–L3 burst fracture; (III) adequate preoperative and postoperative imaging; (IV) history of both emergent and elective procedure; (V) single and multiple vertebral body fractures; (VI) no neurological impairment.
Patients were excluded from the SJ group if they had vertebra plana, pedicle rupture, pedicle diameter <6 mm, spinal canal encroachment ≥50%, vertebral body spread >30%, osteoporotic vertebral fractures, malignant lytic lesions, and burst fractures beyond the T10–L3 levels. Patients were excluded from the PA group if they had a fracture beyond T10–L3 or required circumferential arthrodesis.
After careful patient selection, this study included 54 patients who underwent PA and 47 SJ patients.
Data collection
For each patient in both SJ and PA groups, we collected age, sex, fracture type, fracture level, back pain at admission and last follow-up, operative time (min), discharge time (days), vertebral body height parameters at admission and after SJ implant, pre and post SJ implant posterior wall retropulsion, vertebral kyphosis (VK) and local kyphosis (LK) angles, vertebral body volume at admission and after SJ implant, surgical and medical complication (i.e., the pattern of cement leakage), and length of follow-up (months).
Type of vertebral fractures was defined based on Magerl’s classification (9). Back pain was collected at admission and the last clinical follow-up visit by a 10-point visual analogue scale (VAS). Pre and postoperative imaging consisted of computer tomography (CT) and orthostatic X-ray [anteroposterior (AP) and latero-lateral (LL)] scans of the whole thoracolumbar segment. In the SJ group, imaging was performed just before and after implantation, at one month postoperatively, and at a last radiological follow-up. In both groups, the last radiography examination performed was considered the one useful for parameter estimation. In the PA group, postoperative imaging consisted of orthostatic thoracolumbar X-ray to measure VK and LK angles. In the SJ group, mandatory pre-and postoperative imaging also included volumetric thoracolumbar CT scans for calculating vertebral body parameters.
Height of the vertebral body (VBH) was acquired in three different locations: anterior vertebral body height (anterior VBH), posterior vertebral body height (posterior VBH), and mid-vertebral body height (mid-VBH).
The kyphosis was assessed in two ways: as VK, which is the angle between the superior and inferior plate of the treated vertebra; and LK as the Cobb angle, which is the angle between the superior endplate one level above the treated vertebra and the inferior endplate one level below the treated vertebra.
The vertebral body volume (VB volume) was calculated using dedicated software (Slicer 3D, version 4.10.1, www.slicer.org). Vertebra segmentation was done semi-quantitatively with Slicer 3D, which is a software solution commonly adopted for brain and spine segmentation (10). A 2D segmentation was drawn in an axial, sagittal, and coronal acquisition. These specific sets of acquisitions were used to generate a rough 3D interpolation of the vertebral body. The 3D interpolation was then manually corrected by adding or erasing certain critical areas for proper segmentation (Figure 1).
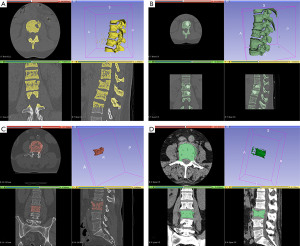
The posterior wall retropulsion (PWR) was measured on pre-and post-procedural CT scans perpendicularly from the posterior wall line on the midsagittal scans. A straight line was drawn on the midsagittal plane from the posterior-inferior corner of the cranial to the posterior-superior corner of the caudal adjacent vertebral bodies, ideally representing the original position of the normal pre-fracture posterior wall of the target level. The distance to the line tangent to the fractured segment was calculated.
Patterns of cement leakage were classified according to Yeom et al. (11) as follows: type B via the basivertebral vein, type S via the segmental vein, and type C through a cortical defect.
Both an experienced Neuroradiologist and Neurosurgeon independently reviewed each patient’s imaging.
Group allocation and SJ technique
In our institution, all of the patients underwent multidisciplinary discussions before being programmed for intervention. Our spine multidisciplinary team comprises experienced neuroradiologists and spine surgeons who review all the presented cases together before concluding. Ideal patients for SJ were considered mostly younger patients with a fresh fracture with wider pedicles (>6 mm), without severe posterior wall retropulsion (<50% of spinal canal encroachment), neurologically intact, and willing to recover a physically active life. Also, elderly patients with comorbidities or unfit for long intervention under general anesthesia were considered good candidates for SJ. Contrarily, in the absence of the aforementioned factors, we were more prone to choose traditional posterior arthrodesis or add a short construct to reinforce the SJ implant. We summarize the patients’ selection criteria in Figure 2.
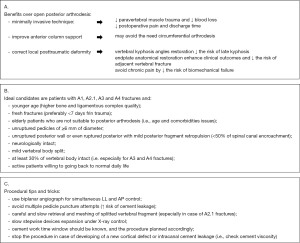
As mentioned above, in a few SJ patients (6), we added short posterior instrumentation (one level above and one level below) to show any possible use of the device alone or in a hybrid form. Furthermore, those were cases from the beginning of our experience in using SJ; thus, those were patients in which we felt it was safer to add posterior instrumentation to make a more solid construction.
In the PA group, we both included short (one level above and on levels below, 20 patients) and long posterior arthrodesis (two-level above and two-level below, 34 patients) to analyze each treatment option commonly adopted for thoracolumbar fractures.
We perform all SJ implants using biplanar angiography [Biplane DSA angiography System Artis Q by Siemens (Siemens Healthcare GmbH, Germany)]. Biplane angiography allows to cut time for fluoroscopy arc positioning and allows for simultaneous lateral and anterior scans. In cases in which SJ was supplemented with percutaneous posterior arthrodesis, the patient was transferred to the Neurosurgery operating room, still under anesthesia, to undergo the procedure.
The implant procedure is performed under general anesthesia with the patient in a prone position. Under X-ray guidance, a thin hollow needle is inserted percutaneously through paravertebral muscles to reach the vertebral body. Then, both pedicles and the vertebral body are carefully remade to create enough space for the SJ implants. After simulating the final position with a template, the SpineJack implant is deployed into the fractured vertebral body and slowly and progressively expanded to restore its final height. Two implants are commonly used on each vertebral body side, which are then locked into the desired expanded position. Furthermore, bone cement (~4 mL) is injected to stabilize the restored vertebra.
The procedure is carried out on outpatients, and patients are discharged after early mobilization (12 h) in the Neurosurgical ward. Patients were mobilized with thoracolumbar bracing.
Statistical analysis
Descriptive statistics were reported as median and range, mean and standard deviation (SD) for continuous variables, and proportion and percentage for categorical variables. Parametric comparisons between PA and SJ groups were performed using Student’s t-test, and nonparametric comparisons were performed using the Mann. Whitney U test. When appropriate, categorical comparisons between the two groups were performed using the Pearson’s chi-squared test and Fischer’s exact test. All statistical tests were two-tailed, and the alpha (α) level was set at 0.05. All analyses were performed using commercially available software (Stata 13.0, StataCorp., College Station, TX, USA).
Results
Sample demographics
After reviewing 196 consecutive patients undergoing vertebral thoracolumbar fracture treatment over 3 years, we eventually identified 101 patients with a type A2, A3, or A4 fracture, from T11 to L3, matching our inclusion criteria. Fifty-four patients were included in the posterior arthrodesis group (PA group) and 47 patients with SJ (SJ group), as shown in Figure 3.
Overall, the median age at presentation was 59 years [interquartile range (IQR): 23–79 years]. The male to female ratio was 1.8:1, and 35.6% of patients were female. The majority of fractures were classified as type A3 (62.7%), followed by A2 (22.6%) and type A4 (13.7%). Fractures were located for the most at L1 (49.5%), followed by T12 (27.7%), L2 (13.9%), and eventually T11 and L3 (5.0% and 4.0%, respectively).
In the PA group, median age was 60 years (IQR: 51–69 years). Male to female ratio was 1.5:1, and 40.7% of patients were female. The majority of fractures were classified as type A3 (62.9%), followed by A4 (20%) and type A2 (14.8%). Half of the fractures were located at L1 (50%), followed by T12 (29.6%), L2 (9.3%), and eventually T11 and L3 (3.7% and 1.9%, respectively). Most patients underwent two levels above and two levels below pedicle screw arthrodesis (62.9%), while the remaining underwent one level above and one level below pedicle screw arthrodesis, with a pedicle screw in the fractured level as well. Median time from admission to treatment was 2.5 days (IQR: 1–4 days).
Median operative time was 110 min (IQR: 59–174 min), and blood loss was 415 mL (IQR: 200–590 mL). Patients were discharged after a median time of 4 days (IQR: 2–5 days). Median follow-up time was 24 months (IQR: 14–36 months).
In the SJ group, the median age was 57 years (41–63 years). Male to female ratio was 2.1:1, and 25.5% were female. The majority of fractures were classified as type A3 (59.6.9%), followed by A2 (23.4%) and type A4 (4.2%). All type A2 fractures fell in the A.2.1 grade as per Magerl’s classification. Forty-seven percent of fractures were located at L1 (50%), followed by T12 (23.4%), L2 (14.9%), and eventually T11 and L3 (4.2% respectively). A double SJ implant was performed for contiguous level fracture in three patients. Median time from admission to treatment was 3 days (IQR: 1–4 days).
Median operative time was 76 min (IQR: 64–93 min), while blood loss was not calculable. There were no cases of medical complications. However, in five cases, we had a mild cement leak towards the intervertebral disc space, and all were type C leakages (i.e., through a cortical defect). The majority (3) of cases happened in A2 fractures, one case in an A4, and one in an A3. None of those leakages had clinical consequences for the patients.
Median follow-up time was 10 months (IQR: 6–15 months). In six patients, percutaneous pedicles screw arthrodesis was adjunct to SpineJack. The median age was 39.5 years (IQR: 35–47 years). There were four males and two females, and four were A2 type fractures and two A3 ones. There were two L2, two L3, one L1, and one T12 fracture. Patients were discharged after a median time of 2 days (IQR: 1–3 days).
Clinico-demographic parameters (i.e., age, sex), fracture type, and level were not significantly different between groups, except for a higher percentage of A4 fractures in the PA group, making the two samples homogeneous in parameters distributions.
Patient demographics and outcomes are summarized in Table 1.
Table 1
| Variables | Arthrodesis | SJ | P value | SJ + short percutaneous arthrodesis |
|---|---|---|---|---|
| N | 54 | 41 | 6 | |
| Sex (female) | 22 | 12 | 0.174 | 2 |
| Aeg, years, median [IQR] (mean ± SD) | 60 [51–69] (57.7±14.9) | 57 [41–63] (53.8±13.3) | 0.087 | 39.5 [35–47] (46±16.8) |
| Fracture type (AO Spine classification) | ||||
| A2 | 8 | 11 | 0.150 | 4 |
| A3 | 34 | 28 | 0.593 | 2 |
| A4 | 12 | 2 | 0.032 | |
| Fracture level | ||||
| T11 | 2 | 2 | 0.774 | |
| T12 | 16 | 11 | 0.765 | 1 |
| L1 | 30 | 19 | 0.722 | 1 |
| L2 | 5 | 7 | 0.265 | 2 |
| L3 | 1 | 2 | 0.413 | 2 |
| Arthrodesis type (No. of level above and below the fracture) | ||||
| 1 | 20 | 6 | ||
| 2 | 34 | |||
| Discharge, median [IQR] | 4 [2–5] | 2 [1–3] | <0.001 | |
| Follow-up time, months, median [IQR] | 24 [14–36] | 10 [6–15] | <0.001 | 7 [6–12] |
SD, standard deviation; SJ, SpineJack.
Clinical and radiological outcomes
In the PA group, the pain went from a preoperative median value of 7.5 [5–10] to a median value of 2 [1–4] postoperatively; while in the SJ group, the pain went from a preoperative median value of 7 [4–10] to a median value of 2 [1–4] postoperatively. There were no statistically significant differences in median postoperative pain outcomes. Median operative time (P<0.001) and median discharge time (P<0.001) were significantly shorter for SJ patients when compared to PA ones.
After SJ implantation, the mean percentage of anterior VBH increase was 20.7%±25.3%, while the mean mid-VBH gain was 25.5%±27.7% and mean posterior VBH gained 8.8%±11.6%, as shown in Figure 4. The mean increase in vertebral volume was 26.2%±33.2% (Figure 5). The VBH and VB volumetric analysis results are summarized in Table 2.

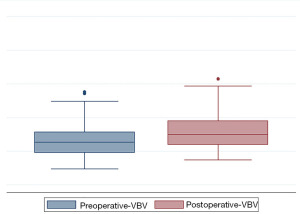
Table 2
| Variables | Overall sample | Sex | Fracture type | |||||
|---|---|---|---|---|---|---|---|---|
| M | F | P value | A2 | A3 | P value | |||
| Mean % of VB height improvement after SJ implant | ||||||||
| Anterior | 20.7±25.3 | 22.1±22.8 | 18.8±31.9 | 0.171 | 22.2±34,3 | 17.7±26.8 | 0.702 | |
| Middle | 25.5±27.7 | 22.1±15.9 | 33.1±44.6 | 0.214 | 25.2±21.7 | 25.2±30.9 | 0.976 | |
| Posterior | 8.8±11.6 | 9.7±10.9 | 6.7±13.0 | 0.432 | 9.1±13.2 | 8.6±10.8 | 0.875 | |
| Mean % of VB volume improvement | 26.2±33.2 | 21.5±24.5 | 37.3±47.2 | 0.136 | 31.9±44.0 | 22.9±25.4 | 0.375 | |
M, male, F, female; VB, vertebral body; SJ, SpineJack.
The mean difference in VK and LK was 3.3°±4.5° and 1.3°±6.3°, respectively, for SJ patients, while it was 2.4°±6.1° (VK) and 2.8°±7.5° (LK), respectively, for PA patients. There were no significant differences in mean kyphosis angles values between the two groups (P value: 0.406 and 0.283, respectively). There were no statistical differences in VK and LK values between PA patients undergoing short or long arthrodesis (P value: 0.400 and 0.652, respectively). A sub-analysis based on follow-up time showed no significant changes in mean VK differences between SJ patients at 6 months (P=0.513), 12 months (P=0.408), and 24 months (P=0.189). The same trend was found for mean differences in LK values in SJ patients after 6 months (P=0.742), 12 months (P=0.754) and at 24 months (P=0.662). In the PA group, subgroup analysis based on follow-up time highlight a slight, even if not significant, trend towards a significant loss of mean LK angles at 24 months (P=0.082).
In the subgroup of patients with SJ implant and additional percutaneous short arthrodesis, although there was a better kyphosis correction, no significant differences were found in mean VK values compared to SJ implant alone (P=0.096 for VK and P=0.077 for LK) or PA (P=0.115 for VK and P=0.262 for LK).
The results of the VBH and VB volumetric analysis are summarized in Table 3.
Table 3
| Variables | Treatment | SJ + short percutaneous arthrodesis | P value* | ||
|---|---|---|---|---|---|
| Arthrodesis | SJ | P value | |||
| Overall sample | |||||
| VK | 2.4°±6.1° | 3.3°±4.5° | 0.406 | 6.5°±2.7° | 0.096 |
| LK | 2.8°±7.5° | 1.3°±6.3° | 0.283 | 6.6°±10.2° | 0.077 |
| A2 | |||||
| VK | −0.9°±3.9° | 1.9°±3.9° | 0.0005 | ||
| LK | 5.0°±5.8° | 1.0°±5.6° | 0.0007 | ||
| A3 | |||||
| VK | 3.4°±6.5° | 4.1°±4.7° | 0.542 | ||
| LK | 2.7°±10.1° | 1.9°±7.5° | 0.661 | ||
*, P value referring to SJ + short percutaneous arthrodesis vs. SJ. SJ, SpineJack.
About 1/3 (27.7%) of the fractures had PWR, of which 69.2% were consequent to A3 fractures, and the remaining (30.7) were in A2 fractures. The mean difference of PWR between pre- and post-SJ implantation was 0.15±0.65 mm. Only in one L2 A3 fracture, we found a positive increase of PWR of 1.4 mm after SJ implantation, with the patients consequently developing right cruralgia. The patient then underwent posterior laminectomy and short arthrodesis. No adjacent above-level fractures were documented over the follow-up period in the whole SJ sample of patients.
After stratifying the analysis by sex and fracture type (A2 versus A3), we found no significant differences in the mean increase of anterior, middle, and posterior VBH parameters between males and females and A2 and A3 procedures. We found a slight increase of vertebral volume gain in A2 fractures after SJ implantation as compared to A3 fractures (31.9%±44.0% vs. 22.9%±25.4%; P=0.375), and a slight increase of postoperative volume in females than males (37.3%±47.2% vs. 21.5%±24.5%; P=0.136).
There was a significant increase in mean VK values for A2 fractures after comparing PA patients and SJ patients (−0.9°±3.9° vs. 1.9°±3.9; P=0.0005). The opposite trend was found for mean LK values for A2 fractures between PA and SJ patients (5.0°±5.8° vs. 1.0°±5.6°; P=0.0007). No differences were found in mean VK and LK values for A3 fractures between PA and SJ patients. We found a slight increase in vertebral body volume in patients ≤35 years old compared to patients older than 35 years old (35.3%±46.6% vs. 24.6%±30.8%; P=0.435).
Discussion
Recently, percutaneous intrasomatic distraction devices, such as SpineJack, have been introduced to overcome the deflation effect of balloon kyphoplasty and allow for minimally invasive stabilization of the vertebral body (5,8,12,13). The SAKOS trial demonstrated the noninferiority of SJ compared to balloon kyphoplasty, with both techniques displaying excellent clinical efficiency and safety and comparable results on daily quality of life. Balloon kyphoplasty has been used to treat thoracolumbar burst fractures (12) alone or as a supplement to screw-rod fixations to provide anterior support (14). In 10–30% of cases, loss of VBH and VB volume from 30 days postoperatively and progressing up to 12 months have been consistently documented (5).
SJ showed better pain relief, mid-VBH restoration, and lower incidence of above adjacent fractures rate (2,13), with cadaveric studies documenting the ability of SJ to maintain this gain after cyclic recompression (14).
SJ provides a vertical expansion with a graduated reduction force, thus remodeling the vertebra on a single axis, stabilizing it with cement, and restoring kyphosis angles. Therapeutic success is mainly related to a meticulous patient selection based on pedicle integrity and limited vertebral body spread (5,7). Implant timing is also crucial since waiting times of more than seven days were frequently associated with the initial fracture consolidation process, preventing device expansion (7).
We thus adopted a progressive approach to SJ implants for post-traumatic fractures, carefully increasing our experience after using it in adjunct to short percutaneous posterior arthrodesis. Combined SJ and short arthrodesis approach patients were significantly younger than SJ alone, and we remarkably improved VK and LK angles compared to SJ alone or PA patients. We believed that a short posterior arthrodesis adjunct should be considered in physically active and young patients (<40 years old). However, our conclusion is based on our preliminary results and the limited sub-sample of patients, limiting our speculations’ generalizability (15).
Contrary to other authors, we found satisfactory radiological outcomes when SJ was used in selected A2 fractures. Overall, cement leakage occurs in 22–31% of cases, and neurological injuries were only described in a few case reports, while the incidence of pulmonary embolisms is estimated between 3.8% and 23% (16). The risk of leakage into the spinal canal may be higher when the fracture affects the posterior vertebral wall, as is A3.1 fractures (approximately in 10% of cases) (16,17). Due to kind of fracture line, the same augmented risk seems to be found in A2 fractures, although clinically irrelevant in all the cases examined (18,19)
SJ and other intrasomatic devices are considered unsuitable for meshing A2 fractures, leading to a higher risk of cement leakage (7) and, consequently, to worse radiological outcomes (7). It may seem reasonable that SJ vertical expansion may only be effective in vertebral remodeling when the distraction is exerted against the comminuted fractures (type A3 or A4). In our monocentric experience, all the A2 fractures were A.2.1 (frontal split fractures), and we did not find such a trend; by exerting significant attention on probe positioning, we recalled VB fragments and bridged them with cement without significant leakages. Also, cement injection and application timing are paramount to avoid further splitting. Therefore, we believe that in selected A2 fractures, VBH parameters and volume gain justify SJ use over the burden of an anterior approach and that operator experience is the critical factor for a successful outcome. Unfortunately, the literature on such fractures and intrasomatic devices deployment is still scarce.
Other paramount measures to take into account during the procedure should be the general condition of the vertebral pedicle and in determining if the posterior wall is disrupted (17). Attention should also be paid to the expansion pressure during the SJ dilatation process, avoiding bursts in cement injection. Expansion should be stopped in case of vertebral cortical bone defect caused by excessive device expansion, and pedicle puncture attempts should be limited to the minimum. Also, the cement work time window should be well handled and cement should be implanted in the sticky stage at low-pressure. Eventually, good radiographic monitoring in lateral and anteroposterior planes is the key to SJ implant (16).
Although non-significant, we found a slight increase in mid-VBH and VB volume in female patients. Considering that there were no differences in age between SJ males and females (51.5 vs. 55.6 years; P=0.357), we believe that fractures in females of menopausal age may be aggravated by undiagnosed underlying mild osteoporosis. Indeed, the SJ distraction force could have been applied more efficiently than males in such patients due to lower bone resistance.
Another feared complication after SJ implant is PWR increase due to cement injection and device spreading, leading to neurological symptoms. However, if SJ is regularly positioned (i.e., in the most anterior position and with convergent trajectories), the force exerted on the posterior wall is negligible, leaving PWR to be progressively pushed back by ligamentotaxis (12).
Eventually, some authors speculated (20,21) that the more stiffened vertebra could lead to above-level fractures over time. The proposed biomechanical reason is that the shifting VB center of gravity more anteriorly along with the secondary stress effects on the above vertebra a large amount of globular cement (22). Also, it may lead to the loss of supporting pressure in the supra-adjacent disc, as the disc ‘expands’ into the collapsed vertebra, resulting in poorer support and weight-bearing for the adjacent vertebra (19,23,24). However, contrary to balloon kyphoplasty, it has been shown that intrasomatic implants could restore the disc pressure in the fractured vertebra, thus explaining the significantly lower incidence of adjacent level fractures seen in long-term studies (5,24).
Unfortunately, despite SJ’s efficacy and long-term outcomes in treating osteoporotic fractures well-reported and proven in the literature, studies regarding post-traumatic fractures are still scarce and biased by a relatively short follow-up, between 12 to 27.6 months (5-7,13). However, radiological outcomes in such studies were stable over the evaluated period without significant changes (7), as also highlighted in our results.
Our results show how SJ had overlapping outcomes in terms of postoperative pain combined with an excellent ability to restore VBH parameters, VB volume (approximately >1/3 of the collapsed vertebra), and a non-inferior ability to restore VK and LK angles without aggravating preexistent PWR.
That is particularly true for VK angles when compared to traditional posterior screw rod arthrodesis, especially for A2 fractures. Besides, despite the small number of cases, we showed how SJ with the adjunct of short percutaneous arthrodesis could improve VK and LK angles in line with those of long arthrodesis constructs (21). Eventually, there were no significant complications, and both operative and discharge times were significantly lower for SJ patients, making the procedure safe and time-effective (7,20,21).
Limitations
The present study’s main limitation was the relatively small number of patients and the relatively short median follow-up time, limiting our results’ generalizability. Also, it is flawed by the inherent limitations of retrospective studies. Some of our patients may have experienced a burst fracture after mild trauma due to underlying undiagnosed mild osteoporosis (i.e., especially in women over 65 years old). Also, we did not collect factors considered independent predictors of vertebral body weakness (i.e., smoking history, medical therapy). For this study, we did not perform a randomization process. Cases allocation was based on our multidisciplinary expertise, clinco-demographic and fracture characteristics of each case. However, this is one of the few studies comparing radiological outcomes between standard posterior arthrodesis techniques, and we believe it is of interest to spine surgeons. Besides, the strict selection criteria made it possible for the two cohorts of patients to be homogenous in terms of sex, age, fracture type, and level distribution.
We also analyzed different arthrodesis techniques or hybrid constructs for SJ patients to be more inclusive of each treatment modality. Each subgroup underwent a sensitivity analysis, and outcomes were shown in results and tables for readers’ clarity.
Uni and multivariate analysis should have been functional in determining clinical and radiological predictors, but due to the relatively small number of patients and the difficulty of accounting for multiple adjusting factors (i.e., outcomes of fracture treatment are the results of many factors acting together), we prefer to work on it after collecting a larger cohort of patients. In the future, further matched prospective and randomized studies with longer follow-up are necessary to elucidate radiological outcomes and the actual risk of adjacent fractures.
Conclusions
Our study is one of the few comparing the clinical and radiological outcomes of the SpineJack implant to traditional posterior arthrodesis (2,7). SJ showed potentially satisfactory outcomes and a safety profile in a selected range of neurologically intact thoracolumbar split or burst fractures. A shorter operative time and negligible blood loss combined with adequate vertebral body angles and diameters restoration are paramount factors for considering SJ as a reasonable adjunct to the spine surgeon armamentarium.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-21-118/rc
Data Sharing Statement: Available at https://jss.amegroups.com/article/view/10.21037/jss-21-118/dss
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-21-118/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-21-118/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (revised in 2013). All the data from patients are digitalized and prospectively collected, in an anonymized database for exclusive medical use. This study is a prospective collection and retrospective analysis of outcomes, ethical approval was waived by “Aulss 2 Marca Trevigiana” internal review board of our Institution. Informed consent was taken and stored from all participants to use medical imaging.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim BG, Dan JM, Shin DE. Treatment of thoracolumbar fracture. Asian Spine J 2015;9:133-46. [Crossref] [PubMed]
- Marcia S, Piras E, Hirsch JA, et al. Efficacy of a Novel Vertebral Body Augmentation System in the Treatment of Patients with Symptomatic Vertebral Body Fractures. Cardiovasc Intervent Radiol 2021;44:289-99. [Crossref] [PubMed]
- Modi HN, Chung KJ, Seo IW, et al. Two levels above and one level below pedicle screw fixation for the treatment of unstable thoracolumbar fracture with partial or intact neurology. J Orthop Surg Res 2009;4:28. [Crossref] [PubMed]
- McAnany SJ, Overley SC, Kim JS, et al. Open Versus Minimally Invasive Fixation Techniques for Thoracolumbar Trauma: A Meta-Analysis. Global Spine J 2016;6:186-94. [Crossref] [PubMed]
- Hartman J, Granville M, Jacobson RE. Treatment of a High-risk Thoracolumbar Compression Fracture Using Bilateral Expandable Titanium SpineJack Implants. Cureus 2019;11:e4701. [Crossref] [PubMed]
- Vanni D, Galzio R, Kazakova A, et al. Third-generation percutaneous vertebral augmentation systems. J Spine Surg 2016;2:13-20. [Crossref] [PubMed]
- Lofrese G, Ricciardi L, De Bonis P, et al. Use of the SpineJack direct reduction for treating type A2, A3 and A4 fractures of the thoracolumbar spine: a retrospective case series. J Neurointerv Surg 2021; Epub ahead of print. [Crossref] [PubMed]
- Baeesa SS, Krueger A, Aragón FA, et al. The efficacy of a percutaneous expandable titanium device in anatomical reduction of vertebral compression fractures of the thoracolumbar spine. Saudi Med J 2015;36:52-60. [Crossref] [PubMed]
- Magerl F, Aebi M, Gertzbein SD, et al. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J 1994;3:184-201. [Crossref] [PubMed]
- Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 2012;30:1323-41. [Crossref] [PubMed]
- Yeom JS, Kim WJ, Choy WS, et al. Leakage of cement in percutaneous transpedicular vertebroplasty for painful osteoporotic compression fractures. J Bone Joint Surg Br 2003;85:83-9. [Crossref] [PubMed]
- Venier A, Roccatagliata L, Isalberti M, et al. Armed Kyphoplasty: An Indirect Central Canal Decompression Technique in Burst Fractures. AJNR Am J Neuroradiol 2019;40:1965-72. [Crossref] [PubMed]
- Noriega D, Marcia S, Theumann N, et al. A prospective, international, randomized, noninferiority study comparing an implantable titanium vertebral augmentation device versus balloon kyphoplasty in the reduction of vertebral compression fractures (SAKOS study). Spine J 2019;19:1782-95. [Crossref] [PubMed]
- Noriega D, Maestretti G, Renaud C, et al. Clinical Performance and Safety of 108 SpineJack Implantations: 1-Year Results of a Prospective Multicentre Single-Arm Registry Study. Biomed Res Int 2015;2015:173872. [Crossref] [PubMed]
- Krüger A, Oberkircher L, Figiel J, et al. Height restoration of osteoporotic vertebral compression fractures using different intravertebral reduction devices: a cadaveric study. Spine J 2015;15:1092-8. [Crossref] [PubMed]
- Walter J, Haciyakupoglu E, Waschke A, et al. Cement leakage as a possible complication of balloon kyphoplasty--is there a difference between osteoporotic compression fractures (AO type A1) and incomplete burst fractures (AO type A3.1)? Acta Neurochir (Wien) 2012;154:313-9. [Crossref] [PubMed]
- Zhang K, Shen Y, Ren Y, et al. Prevention and treatment of bone cement-related complications in patients receiving percutaneous kyphoplasty. Int J Clin Exp Med 2015;8:2371-7. [PubMed]
- Verlaan JJ, Dhert WJ, Verbout AJ, et al. Balloon vertebroplasty in combination with pedicle screw instrumentation: a novel technique to treat thoracic and lumbar burst fractures. Spine (Phila Pa 1976) 2005;30:E73-9. [Crossref] [PubMed]
- Korovessis P, Hadjipavlou A, Repantis T. Minimal invasive short posterior instrumentation plus balloon kyphoplasty with calcium phosphate for burst and severe compression lumbar fractures. Spine (Phila Pa 1976) 2008;33:658-67. [Crossref] [PubMed]
- Muñoz Montoya JE, Torres C, Ferrer ER, et al. A Colombian experience involving SpineJack®, a consecutive series of patients experiencing spinal fractures, percutaneous approach and anatomical restoration 2016-2017. J Spine Surg 2018;4:624-9. [Crossref] [PubMed]
- Kerschbaumer G, Gaulin B, Ruatti S, et al. Clinical and radiological outcomes in thoracolumbar fractures using the SpineJack device. A prospective study of seventy-four patients with a two point three year mean of follow-up. Int Orthop 2019;43:2773-9. [Crossref] [PubMed]
- Hadley C, Awan OA, Zoarski GH. Biomechanics of vertebral bone augmentation. Neuroimaging Clin N Am 2010;20:159-67. [Crossref] [PubMed]
- Renner SM, Tsitsopoulos PP, Dimitriadis AT, et al. Restoration of spinal alignment and disk mechanics following polyetheretherketone wafer kyphoplasty with StaXx FX. AJNR Am J Neuroradiol 2011;32:1295-300. [Crossref] [PubMed]
- Tzermiadianos MN, Renner SM, Phillips FM, et al. Altered disc pressure profile after an osteoporotic vertebral fracture is a risk factor for adjacent vertebral body fracture. Eur Spine J 2008;17:1522-30. [Crossref] [PubMed]



