
The concept of recovery kinetics: an observational study of continuous post-operative monitoring in spine surgery
Introduction
Low back pain (LBP) is a leading cause of disability worldwide, with 540 million people affected around the world at any one time during 2015 (1). In the field of spine surgery, two of the most common presenting complaints are lumbar spinal stenosis (LSS) and discogenic LBP. In LSS, central canal stenosis of the lumbar spine can manifest as neurogenic claudication, pain, numbness and/or fatigue below the gluteal line that is precipitated by walking and alleviated with sitting down or lumbar flexion (2). Discogenic LBP refers to pain originating from the intervertebral discs, and may be caused by disc infection, mechanical torsion injury, or internal disc disruption (most commonly) from degenerative changes over time (3). When conservative treatment options fail, surgical intervention may be indicated, however methodologies of evaluating patient outcomes or surgical efficacy are not standardised in practice.
The current gold-standard for patient assessment in spine surgery surrounds patient-reported outcome measures (PROMs) (4). Data from PROMs provide a useful “snapshot” of the patient’s perception of their disease at a single timepoint. They may be compared preoperatively and at different timepoints postoperatively with arbitrary standards generally reported at the 6 weeks, 3 months, and annual marks; corresponding with follow-up appointments. However due to sampling bias most PROMs may not adequately capture the fluctuations in functional status that occur between each discrete snapshot, and these periods may be more representative of the patient’s capabilities as a whole, varying with mode of administration and psychometric quality (5,6). As most PROMs are often completed by the subjects themselves there is an introduction of innate perceptive biases that limits objective comparisons of disease severity between different patients (7,8).
The recent increased consumption of smart wearable devices (for example smartphones, smartwatches, and activity trackers) capturing a variety of health metrics have established new avenues for patient evaluation (9,10). These may potentially form the basis of a more objective approach to patient assessment and monitoring across a variety of health-related interventions. Devices are often small, unobtrusive, and lightweight, and can be taken home by patients to facilitate continuous everyday monitoring (9). This permits clinicians a novel perspective to assess fluctuations in functional status that occur between the classical snapshots of PROMs, providing detailed information surrounding the progression of postoperative recovery. It is this concept, specifically the pattern of improvement post intervention that we define as “recovery kinetics”. We aim to examine measurements made using smart wearable devices with the broad goal to examine differences when compared with a traditional PROM approach. In this prospective observational single-centre series, we aim to investigate the recovery kinetics of a population of patients undergoing lumbar spine surgery (either lumbar decompression for LSS or lumbar fusion for discogenic LBP) using continuous (preoperatively to 12 weeks postoperatively) and objective (daily step count and distance travelled) data captured with a wrist-based wearable device. We present the following article in accordance with the STROBE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-22-5/rc).
Methods
Study participants
The present observational study had a recruitment period spanning February to June 2019. Patients with either (I) a clinical diagnosis of LSS who were deemed suitable for lumbar decompression, or (II) a clinical diagnosis of discogenic LBP who were deemed suitable for lumbar fusion were recruited from the NeuroSpine Clinic at the Prince of Wales Private Hospital, Randwick, Australia. Recruited participants were categorised into either a Decompression or Fusion group prior to statistical analysis of subjective and objective outcome measures, pre- and post-operatively. Only patients with single level surgery were considered for inclusion. During each patient’s hospital admission, study parameters and risks were discussed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All methods were carried out in accordance with relevant guidelines and regulations of the South-Eastern Sydney Local Health District Ethics Board. Ethics for this study was obtained from the South-Eastern Sydney Local Health District Ethics Board under reference code HREC: 17/184. Written informed consent was obtained from the included participants prior to participation in this study.
Data collection
Each patient completed the Oswestry Disability Index (ODI) preoperatively and at 12 weeks postoperatively. The ODI is a 10-item validated questionnaire for degenerative diseases of the lumbar spine assessing the impact of relevant symptoms across domains of pain, personal care, and daily activities (11-14). In addition, each patient wore a wrist-based wearable device (Mi-Band2, Xiaomi, China) which continuously collected objective physical activity metrics (daily step count and daily distance travelled) in the preoperative week to 12 weeks postoperatively.
Wearable device
Physical activity metrics were assessed using the wrist-based Mi Band 2 (Xiaomi, China) smartwatch. The data captured was transmitted via Bluetooth™ to an AndroidTM or iOS smartphone with the MiFit application (Xiaomi, China). Participants were instructed to sync their wrist-based wearable device to their smartphone application daily and this data was recorded by investigators upon presentation, and at the final post-operative follow-up time-point. The difference in data recording of the accelerometers and observational tests have previously been reported to be within 5%, therefore confirming respective accuracy (15). The same wearable device was used to collect preoperative and postoperative data to negate collection bias between different devices. Compliance was excellent, with staff at enrolment explaining the importance of continuous data collection to patients. This was reinforced at postoperative check-ups, and during routine phone calls from a research practice nurse on weeks 1, 2, and 6. No patient reported forgetting to wear their watch, and no patient recorded 0 steps on any day therefore confirming compliance.
Statistical analysis
Data analyses were performed using SPSS statistical software (version 24.0, IBM, Armonk, New York, USA). Differences between preoperative and postoperative timepoints for participants were assessed paired samples t-tests. Welch’s correction was applied for variables with unequal variance. Normality was assessed using the Shapiro-Wilk test and visual inspection of histograms. Statistical significance was considered for P values <0.05.
Results
A total of 24 participants undergoing lumbar spine surgery were recruited for this prospective observational study. Participants were of mean age, 50±12 years and were classified into either the decompression group (n=12), or fusion group (n=12) depending on their operative procedure. All fusion patients received direct nerve root decompression intraoperatively with fusions performed from both anterior (7 patients) and posterior approaches (5 patients). Excluding any index level instability, fusion patients otherwise had normal spinopelvic parameters. Over the 12-week study period, mean daily step count for all (pooled) participants significantly improved from 4,700 to 7,700 steps per day (P=0.01) while their daily walking distance significantly improved from 3,300 to 5,300 meters per day (P<0.01).
Similar trends were present within groups as step counts for the decompression group increased significantly from 5,000 to 7,300 steps per day (P=0.01) and distance travelled increased significantly from 3,300 to 5,300 meters per day (P=0.02). Improvements in the fusion group were also present with step counts significantly increasing from 4,500 to 8,200 steps per day (P=0.01) and distance travelled from 3,200 to 5,300 meters per day (P=0.07). At this 12-week postoperative time-point period ODI scores also decreased from preoperative levels, from 55 to 38 in decompression group and 45 to 35 in fusion group (Table 1).
Table 1
| Daily step count (steps) | Daily walking distance (m) | Oswestry Disability Index | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preoperative | Postoperative | P | Preoperative | Postoperative | P | Preoperative | Postoperative | |||
| All participants (n=24) | 4,700±2,900 | 7,700±3,900 | 0.013 | 3,300±2,200 | 5,300±3,400 | 0.003 | 50 | 37 | ||
| Subgroup | ||||||||||
| Decompression (n=12) | 5,000±3,600 | 7,300±3,800 | 0.010 | 3,400±2,800 | 5,200±3,500 | 0.020 | 55 | 38 | ||
| Fusion (n=12) | 4,500±2,200 | 8,200±4,100 | 0.014 | 3,200±1,600 | 5,300±3,400 | 0.065 | 45 | 35 | ||
Normally distributed data is represented as (mean ± SD). P values represent significance according to paired-samples (two-tail) t-tests. Preoperative refers to the week preceding spinal surgery and postoperative refers to the 12th week of postoperative recovery.
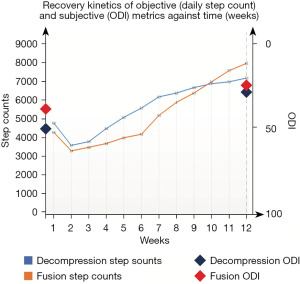
Both decompression and fusion groups affirm a consistent pattern of recovery involving initial decline, followed by accelerated recuperation as measured by an objective mobility metric: mean daily step count. Although overall recovery following both interventions was similar, the groups differ in terms of their recovery kinetics (Table 2, Figure 1). The decompression group improved in daily step count at a rapid initial rate before plateauing in improvements. This contrasts with the fusion group involving a slower rate of recovery initially to preoperative levels, before accelerating in walking capabilities. While the decompression group only reached preoperative levels of daily step count on average by 4.5 weeks postoperatively, fusion group reached this ‘recovery milestone’ on average at 6.5 weeks.
Table 2
| Timepoint (weeks) | Daily step count (mean) | |
|---|---|---|
| Decompression (n=12) | Fusion (n=12) | |
| 1 | 4,900 | 4,400 |
| 2 | 3,700 | 3,400 |
| 3 | 3,900 | 3,700 |
| 4 | 4,600 | 3,900 |
| 5 | 5,200 | 4,200 |
| 6 | 5,700 | 4,500 |
| 7 | 6,300 | 5,300 |
| 8 | 6,500 | 6,000 |
| 9 | 6,800 | 6,500 |
| 10 | 7,000 | 7,100 |
| 11 | 7,100 | 7,700 |
| 12 | 7,300 | 8,100 |
Discussion
The present study demonstrates the application of wearable devices to continuously and objectively track the postoperative recovery of spine surgery patients following fusion and decompression. Over the 12-week study period, mean daily step count for all participants improved from 4,700 to 7,700 steps per day (P=0.013), following an initial dip in total steps taken. The mean daily distance travelled improved from 3,300 to 5,300 meters per day (P=0.003). However, the rate of recovery different between subgroups with decompression group recovering at a faster rate initially than the fusion group, although fusion group recovered to a higher functional level over the longer term. (Figure 1).
Being small, lightweight, and unobtrusive, single-point wearable sensors can be worn in everyday living conditions to allow clinicians to monitor patients continuously. In the setting of spine surgery, this facilitates the construction of a detailed, real-time, objective clinical picture (beyond what is possible with PROMs) of postoperative recovery: Recovery Kinetics. In this study, lumbar spine patients undergoing either decompression or fusion surgery were assessed preoperatively and 12 weeks postoperatively using PROMs. During this timeframe, their activity levels were monitored continuously using a single-point wearable sensor, enabling examination of recovery kinetics. Participants demonstrated increased mean daily step count (4,700 vs. 7,700 steps, P=0.01) and daily walking distance (3,300 vs. 5,300 steps, P=0.003) by the 12th postoperative week, matching reductions in mean ODI scores (50 vs. 37). Additional insight was provided upon examining recovery kinetics and in particular the rate of step count recovery. Although overall improvements were comparable, examination of recovery kinetics revealed distinct patterns when comparing the ‘recovery curves’ of decompression and fusion groups. Most notably, the decompression group experienced a quicker initial rate of recovery compared to the fusion group.
These analyses of recovery kinetics following lumbar decompression and fusion suggest that patient recovery uniquely varies dependent on the operative intervention. Although previous studies have revealed how immediate post-intervention outcomes can differ depending on procedure, no study has yet continuously tracked the postoperative course of recovery. Our findings align with large-scale studies with Martin et al. in a study of 164,527 lumbar fusion patients describing a median hospital stay of four days (16). This was slightly greater than Deyo et al.’s report of 2.7 mean days of hospitalisation following decompression in 21,474 recipients of Medicare in 2007 (17). Similar findings have also emerged from studies assessing recovery in terms of daily step counts with Gilmore et al. reporting a greater total step count in the first postoperative week following lumbar decompression compared to lumbar fusion (14,304 vs. 11,024 steps) (18). Consistent with these results, Stienen et al. reported 48% lower activity levels in the first operative week among patients who underwent a fusion procedure compared to other spinal procedures (1,213 vs. 2,325 steps, P=0.03) (19). The quicker rate of recovery following decompression is likely attributable to a minimal surgical window with exposure of only the lamina. By contrast, fusion requires significant exposure of the lamina, facet joints, transverse processes, and intertransverse spaces and associated bony trauma with instrumentation (20).
In the present study, recovery kinetics were derived from continuous monitoring of walking metrics (mean daily step count), which are well-established determinants of general health and therefore a useful objective outcome measure (21-23). Large-volume cohort studies affirm this notion with Saint-Maurice et al. in a study of adults aged at least 40 (n=4,840) finding a significantly greater incidence (P<0.05) of all-cause mortality amongst those who took less than 4,000 steps/day (76.7 per 1,000 person-years), when compared to those who took between 8–12,000 steps/day (6.9 per 1,000 person-years amongst) (24). Consistent with the literature, we propose that step count (averaged over each day) as a useful long-term measure of health when monitoring patient recovery and rehabilitation following surgery. With further sophistication in newer wearable devices, there is early work in the setting of detailed spatial and temporal gait metrics and their relationship to general and specific measures of health (25,26). These walking metrics such as walking speed, step length, and step time may prove to be more sensitive markers in the examination of recovery kinetics to acute deteriorations, post-operative complications or development of new pathologies (8).
PROMs such as the ODI are the current gold-standard for the assessment of lumbar spine patients (11). In the present study, PROMs were collected at discrete time-points including preoperatively and at the 12-week follow-up timepoint, consistent with common clinical practises. Our findings suggest that continuous and objective mobility metrics collected by a wrist-wearable can capture distinct fluctuations in functional status (as seen in Figure 1) that may occur between these time points; even within the small sample sizes of our decompression (n=12) and fusion (n=12) groups. Examination of the continuous data-stream reflective of patient performance has allowed us to identify the slower initial recovery of our fusion cohort when compared to the decompression cohort.
Although useful in obtaining the impact of the disease on the patient’s daily life, PROMs may not be completely representative of the subject’s functional ability. Stienen et al. found significant variation in the ODI amongst both LBP sufferers (mean, 56.19 vs. 43.44) and healthy pain-free controls (mean, 11.56 vs. 1.36), when comparing higher and lower levels of mental distress (P<0.001) (27). By contrast, objective metrics such as the observed timed up and go (TUG) test faced no such confounding effects in LBP sufferers (mean, 138.4 vs. 116.8, P=0.462) and healthy controls (100.91 vs. 99.65, P=0.897). Herein, lies one of the key advantages of recovery kinetic analyses as valid conclusions and comparisons are made when assessing a patient’s post-operative recovery, free from bias. However, our present study did not assess the presence of any discrepancies between subjective patient questionnaire and objective mobility metrics amongst our cohort of patients undergoing decompression and fusion. These analyses may be warranted in future studies to determine whether the recovery kinetics approach is truly more representative of patient capabilities and function than PROM derived measures alone.
Examining recovery kinetic patterns yields several uses to improve patient care during post-operative recovery and rehabilitation. Previously, authors such as Rushton et al. have alluded to this concept of categorising patients into different “recovery trajectories” depending on rate of improvement in physical function, return to activities of daily living, and development of postoperative complications (28). Although the qualitative nature of data collection using regular (but qualitative) self-reports informed patient perspectives to guide patient-centred recovery and rehabilitation, adherence to patient diaries (of 89.8%) and duration of feasible follow-up (4 weeks postoperatively) were drastically limited (28). Despite low cohort volume, our novel study has demonstrated a distinct pattern of recovery when tracking objective mobility metrics over 12 weeks, in both decompression and fusion patients—an initial decline followed by accelerated recovery (as seen in Figure 1). Wearable-based data-capture, may therefore enable longer durations of clinical follow-up. We recommend the value of medium to long term continuous monitoring to ensure maintenance of recovery performance and guide long-term post-operative rehabilitation. Continuous monitoring may also be sensitive in the early detection of post-operative complications, deterioration and decline as demonstrated by Mobbs et al. (8).
Objective mobility metrics when standardized according to age, sex, and intervention, allow clinicians to assess whether patient progress matches an expected course of recovery. Deviations from these standardized values may alert clinicians that intervention is required, in a similar way that milestone charts are used to monitor child development in paediatrics. This enables a personalised rehabilitation of patient’s according to their unique recovery kinetic trends. Healthcare administrators, insurers, and clinicians alike can benefit from a more efficient allocation of health care resources as patients who are in lower percentiles for their age-, sex-, and intervention-matched recovery kinetics attract timely and sufficient clinical attention.
There are some limitations to this study, and additional work is needed to substantiate the concept of recovery kinetics. Our study only focussed on the recovery kinetics of our study cohorts up to the 12th postoperative week, and future studies could investigate how the recovery kinetics of lumbar spine patients develop beyond this timepoint. Although truly continuous objective data capture with wearables combats sampling bias, this was only minimised and not eliminated by considering weekly average daily step counts in our present study. Future studies could investigate the recovery kinetics of other patient populations. Total hip and knee replacement patients are known to experience gait alterations, and therefore mapping the recovery kinetics of their activity and spatiotemporal gait metrics could be insightful (29-31). Despite being able to distinguish recovery kinetics between decompression and fusion patients, our sample size of 24 is relatively small, and future studies could investigate recovery kinetics in lumbar spine patients using larger sample sizes. Moreover, a larger study may allow stratification of recovery kinetic analyses and construction of standardised recovery kinetic trends based on age and sex.
Conclusions
The recovery kinetics approach of continuous postoperative monitoring provides additional insight to postoperative patient progress. This has uses including tracking rehabilitation goals, the early identification of postoperative complications, and the efficient allocation of health care resources towards patients with relatively poor recovery kinetics.
Acknowledgments
The authors would like to thank the staff from NeuroSpine clinic for assisting with conduct of the project and provision of study materials.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-22-5/rc
Data Sharing Statement: Available at https://jss.amegroups.com/article/view/10.21037/jss-22-5/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-22-5/coif). RJM serves as an Editor-in-Chief of Journal of Spine Surgery, and reports funding of wearable equipment from Jasper Medical Innovations. KR reports administrative support from NeuroSpine Clinic. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545-602. [Crossref] [PubMed]
- Siebert E, Prüss H, Klingebiel R, et al. Lumbar spinal stenosis: syndrome, diagnostics and treatment. Nat Rev Neurol 2009;5:392-403. [Crossref] [PubMed]
- Simon J, McAuliffe M, Shamim F, et al. Discogenic low back pain. Phys Med Rehabil Clin N Am 2014;25:305-17. [Crossref] [PubMed]
- McCormick JD, Werner BC, Shimer AL. Patient-reported outcome measures in spine surgery. J Am Acad Orthop Surg 2013;21:99-107. [Crossref] [PubMed]
- Gagnier JJ, Johnston BC. Poor quality patient reported outcome measures bias effect estimates in orthopaedic randomized studies. J Clin Epidemiol 2019;116:36-8. [Crossref] [PubMed]
- Acosta J, Tang P, Regal S, et al. Investigating the Bias in Orthopaedic Patient-reported Outcome Measures by Mode of Administration: A Meta-analysis. J Am Acad Orthop Surg Glob Res Rev 2020;4:e20.00194.
- DeVine J, Norvell DC, Ecker E, et al. Evaluating the correlation and responsiveness of patient-reported pain with function and quality-of-life outcomes after spine surgery. Spine (Phila Pa 1976) 2011;36:S69-74. [Crossref] [PubMed]
- Mobbs RJ, Katsinas CJ, Choy WJ, et al. Objective monitoring of activity and Gait Velocity using wearable accelerometer following lumbar microdiscectomy to detect recurrent disc herniation. J Spine Surg 2018;4:792-7. [Crossref] [PubMed]
- Henriksen A, Haugen Mikalsen M, Woldaregay AZ, et al. Using Fitness Trackers and Smartwatches to Measure Physical Activity in Research: Analysis of Consumer Wrist-Worn Wearables. J Med Internet Res 2018;20:e110. [Crossref] [PubMed]
- Garg S, Quick HD, Kim EB, et al. Use of Activity Trackers in Orthopaedics. J Am Acad Orthop Surg 2019;27:e859-66. [Crossref] [PubMed]
- Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976) 2000;25:2940-52; discussion 2952. [Crossref] [PubMed]
- Lee CP, Fu TS, Liu CY, et al. Psychometric evaluation of the Oswestry Disability Index in patients with chronic low back pain: factor and Mokken analyses. Health Qual Life Outcomes 2017;15:192. [Crossref] [PubMed]
- Saltychev M, Mattie R, McCormick Z, et al. Psychometric properties of the Oswestry Disability Index. Int J Rehabil Res 2017;40:202-8. [Crossref] [PubMed]
- Cook CE, Garcia AN, Wright A, et al. Measurement Properties of the Oswestry Disability Index in Recipients of Lumbar Spine Surgery. Spine (Phila Pa 1976) 2021;46:E118-25. [Crossref] [PubMed]
- Paradiso C, Colino F, Liu S. The Validity and Reliability of the Mi Band Wearable Device for Measuring Steps and Heart Rate. Int J Exerc Sci 2020;13:689-701. [PubMed]
- Martin BI, Mirza SK, Spina N, et al. Trends in Lumbar Fusion Procedure Rates and Associated Hospital Costs for Degenerative Spinal Diseases in the United States, 2004 to 2015. Spine (Phila Pa 1976) 2019;44:369-76. [Crossref] [PubMed]
- Deyo RA, Mirza SK, Martin BI, et al. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA 2010;303:1259-65. [Crossref] [PubMed]
- Gilmore SJ, Hahne AJ, Davidson M, et al. Physical activity patterns of patients immediately after lumbar surgery. Disabil Rehabil 2020;42:3793-9. [Crossref] [PubMed]
- Stienen MN, Rezaii PG, Ho AL, et al. Objective activity tracking in spine surgery: a prospective feasibility study with a low-cost consumer grade wearable accelerometer. Sci Rep 2020;10:4939. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2-18. [PubMed]
- Betteridge C, Mobbs RJ, Ho D. Proposed objective scoring algorithm for walking performance, based on relevant gait metrics: the Simplified Mobility Score (SMoS™)-observational study. J Orthop Surg Res 2021;16:419. [Crossref] [PubMed]
- Mobbs RJ. Gait velocity (walking speed) is an indicator of spine health, and objective measure of pre and post intervention recovery for spine care providers. J Spine Surg 2020;6:353-5. [Crossref] [PubMed]
- Mobbs RJ, Betteridge C. Daily step count and walking speed as general measures of patient wellbeing. J Spine Surg 2020;6:635-6. [Crossref] [PubMed]
- Saint-Maurice PF, Troiano RP, Bassett DR Jr, et al. Association of Daily Step Count and Step Intensity With Mortality Among US Adults. JAMA 2020;323:1151-60. [Crossref] [PubMed]
- Mobbs RJ, Mobbs RR, Choy WJ. Proposed objective scoring algorithm for assessment and intervention recovery following surgery for lumbar spinal stenosis based on relevant gait metrics from wearable devices: the Gait Posture index (GPi). J Spine Surg 2019;5:300-9. [Crossref] [PubMed]
- Ghent F, Mobbs RJ, Mobbs RR, et al. Assessment and Post-Intervention Recovery After Surgery for Lumbar Disk Herniation Based on Objective Gait Metrics from Wearable Devices Using the Gait Posture Index. World Neurosurg 2020;142:e111-6. [Crossref] [PubMed]
- Stienen MN, Smoll NR, Joswig H, et al. Influence of the mental health status on a new measure of objective functional impairment in lumbar degenerative disc disease. Spine J 2017;17:807-13. [Crossref] [PubMed]
- Rushton A, Jadhakhan F, Masson A, et al. Patient journey following lumbar spinal fusion surgery (FuJourn): A multicentre exploration of the immediate post-operative period using qualitative patient diaries. PLoS One 2020;15:e0241931. [Crossref] [PubMed]
- Bączkowicz D, Skiba G, Czerner M, et al. Gait and functional status analysis before and after total knee arthroplasty. Knee 2018;25:888-96. [Crossref] [PubMed]
- Bahl JS, Nelson MJ, Taylor M, et al. Biomechanical changes and recovery of gait function after total hip arthroplasty for osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage 2018;26:847-63. [Crossref] [PubMed]
- Elkarif V, Kandel L, Rand D, et al. Comparison of the Kinematics Following Gait Perturbation in Individuals Who Did or Did Not Undergo Total Knee Replacement. Appl Sci 2021;11:7453. [Crossref]


