
National trends in the utilization of lumbar disc replacement for lumbar degenerative disc disease over a 10-year period, 2010 to 2019
Introduction
Lumbar degenerative disc disease (LDDD), often accompanied by lumbar back pain (LBP), is a prevalent and debilitating condition with global health, social, and economic impacts (1). For patients with LDDD failing conservative management prior to the development of lumbar disc replacement (LDR), surgical treatment was limited either interbody or posterolateral lumbar fusion (LF). Between 2000–2009, 380,305 patients underwent surgical treatment of LDDD, with a 2.4-fold population adjusted increase over this timeframe (2). After Federal Drug Administration (FDA) clearance in 2004, initial studies regarding LDR showed reductions in short- and long-term complications compared with lumbar fusion in the treatment of LDDD (3).
Lumbar disc replacement has been shown to have non-inferior results and cost savings potential compared to LF in multiple randomized control trials (RCT) (4-7). In a meta-analysis of these RCTs, LDR group had significantly better visual analog scale (VAS) and Oswestry Disability Index (ODI) scores and lower complication rates than the LF group at 2-year post-surgery; no difference was observed in re-operation rates (8). A second meta-analysis found LDR to have improved ODI scores, decreased risk of reoperation and increased likelihood of patient satisfaction compared to LF (9). Multiple studies have shown that LDR may have lower rates of adjacent segment disease (ASD) than LF at up to 5 years (10,11).
Despite positive initial study results, the adoption of LDR as a surgical intervention for LDDD has been low. In the period of 2005–2009, LDR was performed in just 2.7% of surgeries for LDDD (2). Subsequently, LDR utilization decreased more than 80% through 2013 (12). While orthopaedic implant companies continue to develop new LDR implants, limited data on LDR usage and complications are available for recent years. The purpose of this study is to analyze trends in the surgical treatment of LDDD using a representative national database from 2010–2019 to determine recent trends in LDR utilization. We present the following article in accordance with the STROBE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-22-4/rc).
Methods
We performed a retrospective cohort study utilizing a nationally representative publicly available administrative database. Our study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was identified using the National Inpatient Sample (NIS) over the most recent 10-year period with data available (January 1, 2010 to December 31, 2019). The NIS is a nationally representative database developed from all hospitals participating in the Healthcare Cost and Utilization Project (HCUP). The validity of the database is ensured by a multilateral group, the Agency for Healthcare Research and Quality (AHRQ). It comprised data from inpatient hospitalizations from over 40 states derived from billing and discharge information. It is estimated to cover more than 95% of the U.S. population using an estimate of 20% stratified sample of discharges from U.S. hospitals. Of note, outpatient and same day surgical procedures are not included in this database. Discharge weights reported by participating HCUP institutions are used to inform a stratification formula that allows for the estimation of nationally representative statistics (13). The NIS database has been de-identified of any personal health information, and as such this study was deemed exempt by the Institutional Review Board at our institution.
Patients aged 18 years or older who were admitted for LF or LDR with a diagnosis of LDDD were included. Patients were identified using the International Classification of Diseases, 9th Revision (ICD-9) as well as International Classification of Diseases, 10th Revision (ICD-10) diagnosis and procedure codes (Table 1). Number of levels included in surgical fusion was determined by the number of lumbar fusion codes listed, or in the case of ICD-10, the inclusion of specific two-level fusion codes (Table 1). Exclusion criteria included age less than 18 years, three level fusion or surgery, and surgery without the associated diagnosis code for DDD.
Table 1
| Group | ICD-9 | ICD-10 | |
|---|---|---|---|
| Diagnosis | DDD | 812.0, 812.01, 812.03, 812.03, 812.09 | M51.06, M51.16, M51.17, M51.26, M51.27, M51.36, M51.37, M51.86, M51.87 |
| Procedure | LDR | 84.65 | 0SR20JZ, 0SR207Z, 0SR20KZ, 0SR40JZ, 0SR407Z, 0SR40KZ |
| LF | 81.06, 81.07, 81.08 | 0SG0070, 0SG0071, 0SG007J, 0SG00A0, 0SG00AJ, 0SG00J0, 0SG00J1, 0SG00JJ, 0SG00K0, 0SG00K1, 0SG00KJ, 0SG0370, 0SG0371, 0SG037J, 0SG03A0, 0SG03AJ, 0SG03J0, 0SG03J1, 0SG03JJ, 0SG03K0, 0SG03K1, 0SG03KJ, 0SG0470, 0SG0471, 0SG047J, 0SG04A0, 0SG04AJ, 0SG04J0, 0SG04J1, 0SG04JJ, 0SG04K0, 0SG04K1, 0SG04KJ, 0SG1070, 0SG1071, 0SG107J, 0SG10A0, 0SG10AJ, 0SG10J0, 0SG10J1, 0SG10JJ, 0SG10K0, 0SG10K1, 0SG10KJ, 0SG1370, 0SG1371, 0SG137J, 0SG13A0, 0SG13AJ, 0SG13J0, 0SG13J1, 0SG13JJ, 0SG13K0, 0SG13K1, 0SG13KJ, 0SG1470, 0SG1471, 0SG147J, 0SG14A0, 0SG14AJ, 0SG14J0, 0SG14J1, 0SG14JJ, 0SG14K0, 0SG14K1, 0SG14KJ, 0SG3070, 0SG3071, 0SG307J, 0SG30A0, 0SG30AJ, 0SG30J0, 0SG30J1, 0SG30JJ, 0SG30K0, 0SG30K1, 0SG30KJ, 0SG3370, 0SG3371, 0SG337J, 0SG33A0, 0SG33AJ, 0SG33J0, 0SG33J1, 0SG33JJ, 0SG33K0, 0SG33K1, 0SG33KJ, 0SG3470, 0SG3471, 0SG347J, 0SG34A0, 0SG34AJ, 0SG34J0, 0SG34J1, 0SG34JJ, 0SG34K0, 0SG34K1, 0SG34KJ |
DDD, degenerative disc disease; ICD, International classification of diseases; LDR, lumbar disc replacement; LF, lumbar fusion.
The primary outcomes studied included hospitalization cost, length of stay (LOS), non-home discharge destination and index hospitalization complications for patients undergoing LDR versus LF. Hospitalization cost are calculated for each hospitalization record using reported hospitalization charges multiplied by hospital-specific cost-to-charge ratios provided by the AHRQ (14). Hospital costs include all aspects of a given hospital stay—costs of hospital bed, procedures, diagnostic studies, physician fees—and an itemized breakdown was not available. Costs were inflation-adjusted based on admission month using rates from United States (US) Bureau of Labor Statistics and described in December 2019 U.S. dollars (15). Index hospitalization complications were defined using ICD-9 and ICD-10 codes reported in (16) with the addition of neurologic complication codes (ICD-9 997 and 349.31; ICD-10 G97 and G96). The following complication categories were analyzed: cardiac complications, neurologic complications, myocardial infarction, cerebrovascular accident (CVA), respiratory complications, pneumonia (PNA), pulmonary embolism (PE), other pulmonary complications, deep vein thrombosis (DVT), acute kidney injury, wound complications, post-operative blood transfusions or any in-hospital complications.
All reported patient demographics and hospital characteristics were summarized and analyzed with regard to primary and secondary outcomes. Patient demographics include age (in years), sex (male and female), reported race and ethnicity (classified as white, Black, Hispanic, and other), and primary hospitalization payor or insurer (Medicare, Medicaid, private, and self-pay). The following hospital characteristics are available in NIS: hospital type (classified as urban non-teaching, urban teaching, and rural), hospital size based on number of beds (reported as large, medium, and small), and US census region (Northeast, Midwest, South, and West). Overall level of patient medical complexity and underlying comorbid conditions was quantified using the Elixhauser Comorbidity Index (ECI), acomposite score of 30 diagnostic groups that has been shown to be predictive of mortality in orthopaedic patients and have better predictive accuracy than other comorbidity indices (17). ECI scores were generated using the comorbidity package in R (18) incorporating all associated ICD diagnostic codes for a given admission (18).
Statistical analysis
Reported sample sizes represent national estimates as NIS discharge-level weights were incorporated. Descriptive statistics were used to describe both baseline characteristics and outcome parameters within each comparison group. Analysis was done using a two tailed Student’s t-test after ensuring normal distributions. For skewed, nonparametric distributions, continuous variables are presented as median (interquartile range), and analyzed using the Wilcoxon rank-sum test. Chi-squared tests were used for categorical analysis. In longitudinal analyses of annual estimates of fixation method utilized, we performed trend using univariate linear or logistic regression with year as a linear predictor. Propensity score matching (PSM) of LDR to LF patients was performed to compare primary outcomes between groups using the MatchIt package in R (19,20). A propensity score multivariate logistic regression model predicting treatment type (LDR vs. LF) was created using pre-specified patient and hospital-level variables. These included: patient age, sex, ECI, hospital type, hospital size, insurance status, and zip code-based income quartile. Medical history variables were also included in the model: history of CHF, cardiac arrythmia, pulmonary hypertension, chronic pulmonary disease, essential hypertension, diabetes, obesity, coagulopathy and alcohol abuse. PSM was selected over multivariate regression or inverse-probability of treatment weighted analysis given the relatively low rate of LDR and the interest in studying the effects of this procedure in a smaller group of LDR-eligible patients, rather than the population at large (17,19,21). For PSM, matching was performed with a ration of 1:2 without replacement, and a caliper was utilized with a width of 0.2 times the standard deviation of all logit propensity scores (19,20). Post-matching balance of covariates was evaluated using standardized mean differences with a threshold of 10% (22). Patients with missing covariates were excluded from PSM analysis. Statistical significance was defined as P<0.05. Statistical analyses were performed using R 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
A total of 505,209 patient records for one of two level LDR or LF procedures were identified, equating to 2,514,854 patients (Figure 1). After removing patients not meeting inclusion criteria, a total of 228,688 patient entries were identified, equating to an estimated 1,137,170 cases performed nationally during the study period. A total of 1,129,121 were LF cases (99.3%) compared to 8,049 LDR cases (0.7%), with 364,637 (32.3%) and 712 (8.8%) comprising two-level surgeries. Summary statistics for each cohort are presented in Table 2. The proportion of female patients was significantly lower for LDR patients (42.5% vs. 52.9%, P<0.001), as was the mean age (41.2 vs. 57.1 years, P<0.001) and mean level of medical comorbidity (ECI score 0.88 vs. 1.80, P<0.001). LDR patients were also more likely to have procedures paid for by private insurance (55.8% vs. 43.7%, P<0.001) and reside in higher income areas (top quartile 30.0% vs. 23.2%, P<0.001). White patients were less likely to undergo LDR, while Hispanic patients were more likely (P<0.001, Table 2).

Table 2
| Variable | LF (n=1,129,121) | LDR (n=8,049) | P |
|---|---|---|---|
| Two-level | 364,637 (32.3%) | 712 (8.8%) | <0.001 |
| Sex, female | 597,098 (52.9%) | 3,402 (42.3%) | <0.001 |
| Age, years | |||
| Mean (SD) | 57.1 (13.8) | 41.2 (10.4) | <0.001 |
| <45 | 222,932 (19.7%) | 5,149 (64%) | <0.0011 |
| 45–59 | 383,482 (34%) | 2,503 (31.1%) | |
| 60+ | 522,656 (46.3%) | 369 (4.6%) | |
| ECI, mean (SD) | 1.80 (1.47) | 0.88 (1.08) | <0.001 |
| Payer | |||
| Medicaid | 75,366 (6.7%) | 560 (7%) | <0.0011 |
| Medicare | 428,925 (38%) | 453 (5.6%) | |
| Private | 492,912 (43.7%) | 4,476 (55.6%) | |
| Self-pay | 8,741 (0.8%) | 228 (2.8%) | |
| Other | 121,487 (10.8) | 2,294 (28.5%) | |
| Race | |||
| White | 874,010 (82.1%) | 5,842 (77.9%) | <0.0011 |
| Black | 80,337 (7.5%) | 491 (6.5%) | |
| Hispanic | 68,734 (6.5%) | 713 (9.5%) | |
| Other | 42,118 (4%) | 457 (6.1%) | |
| Income, quartile | |||
| 0–25% | 259,459 (23.4%) | 1,305 (16.8%) | <0.0011 |
| 25–50% | 297,463 (26.8%) | 1,861 (24%) | |
| 50–75% | 295,703 (26.7%) | 2,257 (29.1%) | |
| 75–100% | 25,6897 (23.2%) | 2,323 (30%) | |
| Hospital type | |||
| Rural | 58,031 (5.2%) | 263 (3.3%) | <0.0011 |
| Urban non-teaching | 400,059 (35.5%) | 3,218 (40%) | |
| Urban teaching | 667,667 (59.3%) | 4,563 (56.7%) | |
| Hospital size | |||
| Large | 587,243 (52.2%) | 3,723 (46.3%) | <0.0011 |
| Medium | 295,771 (26.3%) | 2,645 (32.9%) | |
| Small | 242,743 (21.6%) | 1,676 (20.8%) | |
| LOS, mean (SD) | 3.51 (2.73) | 2.59 (1.84) | <0.001 |
| Cost (USD), mean (SD) | 35,866 (22,924) | 29,837 (17,316) | <0.001 |
| Discharge to Facility | 170,769 (15.2%) | 183 (2.3%) | <0.001 |
1, P value corresponds to chi-squared test for table including rows immediately below. ECI, Elixhauser comorbidity index; LDR, lumbar disc replacement; LF, lumbar fusion; SD, standard deviation; LOS, length of stay.
Longitudinal analysis
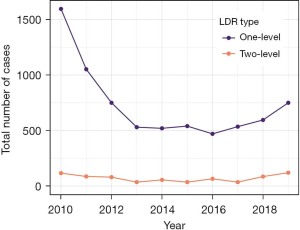
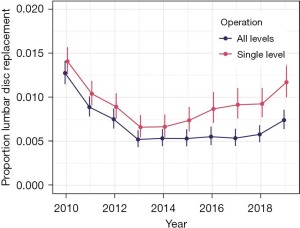
A total of 1,712 LDR cases were performed in the USA in 2010, and this number decreased to 565 by 2013 (Figure 2). The total number of LDR cases annually was roughly constant through 2017 at 570 cases. Annual numbers increased slightly to 680 in 2018 and continued to increase in 870 in 2019, which was similar to the 2012 total of 830. When both one and two-level procedures are analyzed together, the proportion of LDR cases decreased from 1.27% in 2010 to 0.52% in 2013 (P<0.001), stayed constant through 2018 (0.58%, P=0.43 for trend) and increased slightly in 2019 (0.74%, P=0.03, Figure 3). The proportion of two-level procedures increased significantly over the study period from 15.7% in 2010 to 45.5% in 2019 (P<0.001 for trend). As a result, the relative number of single-level procedures decreased. When single-level procedures were analyzed in isolation, the proportion of LDR cases decrease from 1.41% in 2010 to 0.66% in 2013 (P<0.001), but then increased gradually to 1.17% in 2019 (P<0.001 for trend).
Healthcare utilization analysis
Propensity score matching was performed to balance groups using available independent variables. A total of 1,343 single-level LDR patient records were matched to 2,686 single-level LF patient records, and 141 two-level LDR patient records were matched to 282 two-level LF patient records. Of single-level patients, 86 and 7,310 records were missing cost data and 4 and 696 patients were missing discharge data for LDR and LF patients respectively. Of two-level patients, 5 and 1,897 records were missing cost data and 0 and 547 patients were missing discharge data for LDR and LF patients respectively. No patients were missing LOS or index hospitalization complication data.
Single-level LDR patients has significantly shorter LOS (2.45 vs. 3.10 days, P<0.001), lower likelihood of discharge to facility (1.7% vs. 3.4%, P=0.003), and lower costs overall ($27,714 vs. $32,242, P<0.001) compared to matched LF patients (Table 3). When two-level patients were analyzed, there were no differences with regard to LOS and discharge to facility (P=0.38 and 0.21, respectively), while overall costs were significantly higher for LDR patients compared to LF ($53,270 to $44,721, P=0.005).
Table 3
| Outcome | LDR | LF | RR/difference | 95% CI | p |
|---|---|---|---|---|---|
| One-level | (n=1,343) | (n=2,686) | |||
| Discharge to facility | 1.7% | 3.4% | 0.51 (RR) | (0.32, 0.8) | 0.003 |
| LOS | 2.45 | 3.10 | −0.65 (difference) | (−0.76, −0.53) | <0.001 |
| Cost (Dec 2019 USD) | 27,714 | 32,242 | −4,529 (difference) | (−5,581, −3,477) | <0.001 |
| Two-level | (n=141) | (n=282) | |||
| Discharge to facility | 9.9% | 7.4% | 1.33 (RR) | (0.69, 2.56) | 0.38 |
| LOS | 3.95 | 3.64 | 0.31 (difference) | (−0.18, 0.80) | 0.21 |
| Cost (Dec 2019 USD) | 53,270 | 44,721 | 8,549 (difference) | (2,507, 14,591) | 0.005 |
LDR, lumbar disc replacement; LF, lumbar fusion; LOS, length of stay; RR, relative risk; USD, United States dollar.
Inpatient complication analysis
The same matched cohorts were utilized to compare complications occurring during their index admission. Single-level LDR patients has significantly lower rates of any complication (7.0% vs. 13.2%, P<0.001), blood transfusion (3.1% and 8.1%, P<0.001) and neurologic complication (3.0% vs. 4.8%, P=0.006, Table 4). There were no differences with regard to VTE, pneumonia, renal, would or pulmonary complications. There were no significant differences between two-level LDR and LF patients (Table 4).
Table 4
| Complication | One-level | Two-level | |||||
|---|---|---|---|---|---|---|---|
| LDR | LF | P value | LDR | LF | P value | ||
| Any complication | 7.0% | 13.2% | <0.001 | 20.6% | 13.5% | 0.060 | |
| Transfusion | 3.1% | 8.1% | <0.001 | 13.5% | 8.5% | 0.114 | |
| Neurologic | 3.0% | 4.8% | 0.006 | 7.1% | 5.3% | 0.468 | |
| VTE | 0.4% | 0.1% | 0.172 | 1.4% | 0.4% | 0.258 | |
| Pneumonia | 0.8% | 0.7% | 0.599 | 1.4% | 1.1% | 0.752 | |
| Renal | 0.4% | 0.3% | 0.695 | 2.8% | 0.7% | 0.108 | |
| Wound | 0.7% | 0.8% | 0.802 | 0.7% | 1.1% | 0.725 | |
| Pulmonary | 2.8% | 2.8% | 0.946 | 5.0% | 4.6% | 0.872 | |
| Mortality | 0.0% | 0.0% | 1.000 | 0.0% | 0.0% | 1.000 | |
LDR, lumbar disc replacement; LF, lumbar fusion; VTE, venous thromboembolism.
Discussion
Over the most recent decade with data available, the utilization of LDR has remained low in the United States. The number of annual LDR cases decreased significantly from 1,712 in 2010 to 565 in 2013 and subsequently remained roughly constant until increasing slightly in the most recent years to 870 in 2019. LDR patients differed significantly from average LF patients with regard to age, level of medical comorbidity, insurance type and income level. Compared to matched LF patients, patients undergoing single-level LDR had lower hospital hosts, shorter lengths of stay and fewer index hospitalization complications, such as transfusions. These differences were not significant for two-level LDR patients, who had significantly increased costs compared to two-level LF patients.
The results of our analysis follow those found by Saifi et al. in which the utilization of primary LDR was found to decrease 86% from the first full year following FDA approval in 2005 until 2013 (12). Our analysis demonstrates a significant decrease in the percent and gross utilization of primary LDR from the beginning of our study period in 2010 (1.27%) up to 2013 (0.52%), the latest year in past studies. The utilization of LDR then remained relatively constant before rising in 2019 to 0.74% at the end of the study period. This would mark the first significant rise in LDR utilization since its approval in the United States. Despite limited adoption and insurance coverage new models have been approved for LDR use (ActivL artificial disc in June 2015), and two level LDR was FDA-approved for the ProDisc-L from L3-S1 in April 2020, and general support from the Society for the Advancement of Spine Surgery was given in a 2021 position statement (23). Of note a non-trivial proportion of two-level LDRs were performed over the study period (8.8%) before FDA-approval.
In our literature review, we were unable to find any studies demonstrating worse long-term outcomes in LDR patients or any public recalls of LDR implants explaining the continued decline in utilization of LDR in the early 2010’s despite multiple favorable RCTs and prospective studies (4-7,24). Suggested reasons for this low utilization include the multiple counterindications for LDR including high pelvic incidence, translational deformity (spondylolisthesis), and poor bone quality (25). In addition, lack of insurance coverage as well as surgeon familiarity may continue to limit the number of LDR procedures performed (26). These reasons are supported by a survey of 565 spine surgeons in which 55% were concerned about long-term complications and 53% were concerned about the technical challenges of revision (27). In addition, 65% reported a lack of insurance coverage for L-TDR in their region, with 11 of 14 major insurers not covering LDR. This supports our findings that LDR patients are younger, healthier (lower ECI score), more likely to have private payers, and more likely to live in higher income areas than LF patients.
We report that single level LDR had a significantly lower overall complication rate than single-level LF patients (7.0% vs. 13.2%) on matched analysis, with a lower rate of blood transfusion as well (3.1% vs. 8.1%). These rates are similar to those found in a meta-analysis of six LDR RCTs showing a 5.8% overall complication rate in LDR patients compared to 10.8% in LF patients (8). We also found lower LOS, likelihood of discharge to facility, and lower overall costs in single-level LDR patients matched to LF patients. While similar to a 2005–2006 NIS study, this suggests that these differences have persisted since LDRs approval (28). Results from an RCT by Fritzell et al. showed no difference in hospitalization costs between LDR and LF; however, LDR was less expensive when re-operations were incorporated into the analysis (7). Operation(s) on adjacent segments presents an additional cost consideration for LDR and LF. Multiple studies have shown LDR to have a lower rate of adjacent segment disease on long-term follow up and reduced frequency of reoperation and surgery at adjacent levels (10,11,29). Other studies show comparable rates of reoperation between LDR and LF (24,28).
The present study is not without its limitations. First, the authors recognize the inherent weaknesses in a large database study including potential for errors in coding and data entry. The transition to using ICD-10 codes in October 2015 was likely associated with increased variations in coding as new norms were being established. The comparison group of LF patients is highly variable, and contains patients undergoing different approaches (anterior, lateral, posterior) and types of fusion (interbody, posterolateral fusion). Furthermore, this group may contain some patients with additional diagnoses that contraindicate LDR, such as spondylolisthesis. The current inclusion criteria were utilized to make the study directly comparable to prior work on this subject. This study did not evaluate any short- or long-term outcomes following surgical admission because the NIS does not include readmission data. Thus, we are not able to comment on differences in revision surgeries in the years after initial LDR despite this being a major cause of concern among surgeons. Furthermore, no information on functional outcomes or back pain scores is available for analysis. As is the case with any propensity score matched analysis, we cannot control for bias in unmeasured variables between groups—such as LDDD severity, pre-operative function and activity level, global spinal alignment issues, surgeon experience, access surgeon availability—and it is possible that there is some selection bias that remains unaddressed as there are only limited numbers of covariates available in the NIS. Lastly, information regarding surgical details such as implants used, procedure duration, intraoperative complications, and blood loss were unavailable in the NIS. Finally, while this is the largest study on LDR procedures in the United States, the number of procedures remains small, and estimates are subject to uncertainty. Despite these limitations, this study has numerous advantages including the large, nationally representative sample and broad time horizon, including the most recent available data.
In conclusion, the number of LDRs performed in the United States declined over the 2010–2013 period before rising in 2019. Overall utilization of LDR for LDDD remains low with 870 cases performed in 2019, accounting for 0.74% of surgical procedures performed for LDDD that year. Adoption of LDR is low relative to LF due to multiple factors including concerns regarding the long-term durability of the procedure, as well as technical difficulties associated with revision surgery in the anterior lumbar spine. However, LDR demonstrated some benefits over LF during the index hospitalization in our study. LDR patients experienced lower overall surgical complication rates with a significant difference in the necessity for blood transfusion compared to matched LF patients. The lower costs, LOS, and facility discharge rates coupled with comparable long-term outcomes described in prior medium-term studies suggest single-level LDR as a viable alternative to single-level LF in carefully selected healthy, younger patients. Further long-term studies will help demonstrate value associated with reduced ASD to improve coverage by major insurers and characterize any potential unique challenges and complications associated with LDR revision—two likely barriers to LDR adoption in the US.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-22-4/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-22-4/coif). EL reports receiving an AOSpine travel grant to participate in editorial duties for Global Spine Journal. DYP reports receiving royalties from Seaspine and consulting fees from Seaspine, Globus, Nuvasive and Amplify Surgical. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Teraguchi M, Yoshimura N, Hashizume H, et al. Prevalence and distribution of intervertebral disc degeneration over the entire spine in a population-based cohort: the Wakayama Spine Study. Osteoarthritis Cartilage 2014;22:104-10. [Crossref] [PubMed]
- Yoshihara H, Yoneoka D. National trends in the surgical treatment for lumbar degenerative disc disease: United States, 2000 to 2009. Spine J 2015;15:265-71. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2-18. [PubMed]
- Gornet MF, Burkus JK, Dryer RF, et al. Lumbar disc arthroplasty with Maverick disc versus stand-alone interbody fusion: a prospective, randomized, controlled, multicenter investigational device exemption trial. Spine (Phila Pa 1976) 2011;36:E1600-11. [Crossref] [PubMed]
- Zigler J, Delamarter R, Spivak JM, et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine (Phila Pa 1976) 2007;32:1155-62; discussion 1163. [Crossref] [PubMed]
- Guyer RD, McAfee PC, Banco RJ, et al. Prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: five-year follow-up. Spine J 2009;9:374-86. [Crossref] [PubMed]
- Fritzell P, Berg S, Borgström F, et al. Cost effectiveness of disc prosthesis versus lumbar fusion in patients with chronic low back pain: randomized controlled trial with 2-year follow-up. Eur Spine J 2011;20:1001-11. [Crossref] [PubMed]
- Wei J, Song Y, Sun L, et al. Comparison of artificial total disc replacement versus fusion for lumbar degenerative disc disease: a meta-analysis of randomized controlled trials. Int Orthop 2013;37:1315-25. [Crossref] [PubMed]
- Zigler J, Gornet MF, Ferko N, et al. Comparison of Lumbar Total Disc Replacement With Surgical Spinal Fusion for the Treatment of Single-Level Degenerative Disc Disease: A Meta-Analysis of 5-Year Outcomes From Randomized Controlled Trials. Global Spine J 2018;8:413-23. [Crossref] [PubMed]
- Zigler JE, Glenn J, Delamarter RB. Five-year adjacent-level degenerative changes in patients with single-level disease treated using lumbar total disc replacement with ProDisc-L versus circumferential fusion. J Neurosurg Spine 2012;17:504-11. [Crossref] [PubMed]
- Ren C, Song Y, Liu L, et al. Adjacent segment degeneration and disease after lumbar fusion compared with motion-preserving procedures: a meta-analysis. Eur J Orthop Surg Traumatol 2014;24:S245-53. [Crossref] [PubMed]
- Saifi C, Cazzulino A, Park C, et al. National Trends for Primary and Revision Lumbar Disc Arthroplasty Throughout the United States. Global Spine J 2018;8:172-7. [Crossref] [PubMed]
- Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) Introduction to the NIS 2016. Available online: www.hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2016.jsp. 2021.
- Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) Cost-to-Charge Ratio Files 2020. Available online: www.hcup-us.ahrq.gov/db/ccr/costtocharge.jsp
- United States Bureau of Labor Statistics. Average Price Data. Available online: https://download.bls.gov/pub/time.series/cu/cu.data.1.AllItems. 2021.
- Upfill-Brown A, Hart CM, Hsiue PP, et al. Revision Total Hip Arthroplasty in Solid Organ Transplant Patients: A Propensity Score-Matched Cohort Study for Aseptic and Infected Revisions. Arthroplast Today 2022;14:6-13. [Crossref] [PubMed]
- Menendez ME, Neuhaus V, van Dijk CN, et al. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res 2014;472:2878-86. [Crossref] [PubMed]
- Gasparini A. comorbidity: An R package for computing comorbidity scores. Journal of Open Source Software 2018;3:648. [Crossref]
- Ho DE, Imai K, King G, et al. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. Journal of Statistical Software 2011;42:1-28. [Crossref]
- Ho DE, Imai K, King G, et al. Matching as Nonparametric Preprocessing for Reducing Model Dependence in Parametric Causal Inference. Political Analysis 2007;15:199-236. [Crossref]
- Austin PC, Stuart EA. The performance of inverse probability of treatment weighting and full matching on the propensity score in the presence of model misspecification when estimating the effect of treatment on survival outcomes. Stat Methods Med Res 2017;26:1654-70. [Crossref] [PubMed]
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083-107. [Crossref] [PubMed]
- Schroeder GD, Vaccaro AR, Divi SN, et al. 2021 Position Statement From the International Society for the Advancement of Spine Surgery on Cervical and Lumbar Disc Replacement. Int J Spine Surg 2021;15:37-46. [Crossref] [PubMed]
- David T. Long-term Results of One-Level Lumbar Arthroplasty. Spine (Phila Pa 1976) 2007;32:661-6. [Crossref] [PubMed]
- Salzmann SN, Plais N, Shue J, et al. Lumbar disc replacement surgery-successes and obstacles to widespread adoption. Curr Rev Musculoskelet Med 2017;10:153-9. [Crossref] [PubMed]
- Awe OO, Maltenfort MG, Prasad S, et al. Impact of total disc arthroplasty on the surgical management of lumbar degenerative disc disease: Analysis of the Nationwide Inpatient Sample from 2000 to 2008. Surg Neurol Int 2011;2:139. [Crossref] [PubMed]
- Hart RA, DePasse JM, Daniels AH. Failure to Launch: What the Rejection of Lumbar Total Disk Replacement Tells us About American Spine Surgery. Clin Spine Surg 2017;30:E759-64. [Crossref] [PubMed]
- Kurtz SM, Lau E, Ianuzzi A, et al. National revision burden for lumbar total disc replacement in the United States: epidemiologic and economic perspectives. Spine (Phila Pa 1976) 2010;35:690-6. [Crossref] [PubMed]
- Harrop JS, Youssef JA, Maltenfort M, et al. Lumbar adjacent segment degeneration and disease after arthrodesis and total disc arthroplasty. Spine (Phila Pa 1976) 2008;33:1701-7. [Crossref] [PubMed]


