
Prone thoracic endovascular aortic repair via the popliteal artery for inadvertent vascular injury during spondylectomy: a case report
Highlight box
Key findings
• Novel technique of thoracic endovascular aortic repair (TEVAR) via the popliteal artery in the prone position is a viable and safe alternative in repairing inadvertent vascular injury during posterior spinal surgery.
What is known and what is new?
• There are no cases of TEVAR in the prone position or TEVAR via the popliteal artery described in the literature.
• This case describes TEVAR in the prone position via the popliteal artery for inadvertent vascular injury during posterior spinal surgery.
• This technique bypasses repositioning/transferring of the patient for angiography, which can lead to faster control of haemorrhage.
What is the implication, and what should change now?
• Surgeons should be aware such approach exists and can be used as a viable alternative to the traditional femoral surgical cutdown access for vascular injuries during posterior spinal surgery.
Introduction
Vascular injury as a complication of spinal surgery is a rare but devastating complication with reported incidence between 0.01% to 3%, associated with high mortality up to 65% (1,2). Traditional methods to address this involve endovascular repair after patient repositioning and transfer to the angiographic suite with concomitant delay (2,3). Here we describe a novel technique where endovascular access was achieved via the popliteal artery to avoid patient repositioning, with successful deployment of a thoracic endovascular aortic repair (TEVAR) graft in the prone position. This article is written following the CARE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-23-17/rc) (3).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
History and examination
A 63-year-old patient with a history of metastatic neuroendocrine tumour presented with 10 days of worsening back pain following a fall. Magnetic resonance imaging (MRI) revealed a T11 lesion with posterior extension into the spinal canal causing spinal cord compression and myelomalacia (Figure 1). The patient had previously completed a course of radiotherapy to this known T11 metastatic deposit and vertebroplasty had also been performed to halt the progressive kyphotic deformity caused by this lesion. Staging scans demonstrated isolated disease at T11 and a decision was made for T11 en bloc spondylectomy and T9 to L2 posterior instrumented fusion.

Intraoperative course
The patient was positioned prone with arms raised, and a mobile C-arm imaging system was used to aid the placement of the screws. After instrumentation of T9-L2 the posterior elements of T11 were removed along with the rib heads of T11 and 12. During ventral dissection of the T11 vertebral body and attempted separation of the aorta from the vertebral surface, sudden arterial bleeding filled the surgical field. The on-call vascular surgeon was contacted. Primary repair of the vascular injury was not possible given the intact vertebral column which obscured direct access to the aorta. The patient remained hypotensive and tachycardic precluding immediate transfer to the angiography suite. A decision was made by the vascular surgeons to access the right popliteal artery in the prone position in order to deploy a stent for TEVAR.
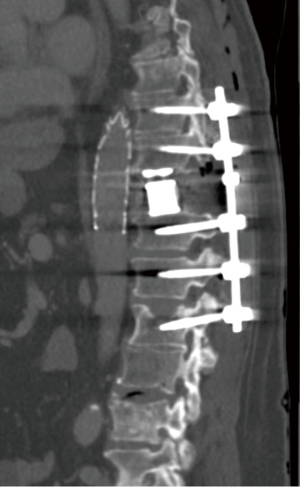
The standard angiography set was prepared. The right popliteal fossa was prepped and a cannula was inserted into the right popliteal artery using ultrasound guidance, and an 8 French size catheter was upsized to 22 French over a stiff wire. The intraoperative X-ray machine used for the spinal hardware placement was also able to perform digital subtraction angiography (DSA). The coeliac artery was identified on angiography, and whilst it was difficult to appreciate the vascular injury on DSA, it was adequate to locate the level of the spondylectomy and presumed location of arterial injury. Ensuring the guidewire passed the popliteal artery into the femoral artery, and accounting for the inverted anatomy on angiography, a 34 mm × 34 mm × 150 mm Ctag (Gore) TEVAR graft was deployed under radiological guidance using bony landmarks alone (Figure 2). Following this, the spinal wound was unpacked and the previous arterial bleeding was noted to have ceased. Spondylectomy was then able to be completed (Figure 3). The thoracic aorta could then be directly visualised where 2 avulsed intercostal arteries were controlled using 6-0 sutures and liga clips. Spinal reconstruction was then completed with the patient subsequently transferred to the intensive care unit for continued resuscitation and inotropic support. A follow up computed tomography (CT) angiogram was performed showing adequate placement of the TEVAR graft (Figure 4).

Time from identification of injury to completion of TEVAR was estimated at 90 minutes. Exact timing from identification of injury to initiation of TEVAR was not measured, but approximately at 60 minutes. Blood loss was estimated at 800 mL during the vascular injury, 4 units of packed red blood cells were transfused. The patient was extubated day 1 post op with intact lower limb neurology. There were no complications at the popliteal access site. Postoperative course included 3 weeks of inpatient stay, including 9 days at a rehabilitation centre and followed by further 3 weeks of outpatient rehabilitation. The patient was discharged home walking independently with a 4-wheeled-walker, and re-commenced chemotherapy at 2-month for disease progression.
Discussion
This case represents the first described prone TEVAR via the popliteal artery for iatrogenic arterial injury during posterior thoracic spinal surgery. Vascular injuries associated with spinal surgery often present in a delayed fashion, more commonly associated with pedicle screw mal-placement (4). However, major intraoperative haemorrhage can occur due to acute aortic or branch injury (5,6). Vascular injuries during en bloc resection of spinal tumours can be encountered during blunt dissection anterior to the vertebral body, epidural plexus manipulation, specimen removal, or at the tumour margin (5,7). Whilst it is relatively uncommon, representing up to 7.3% of all complications following en bloc resection, it accounts for 41% of complication related mortality, the leading cause of complication related death. This is due to the inability to perform arterial repair in an operative field obstructed by the spinal column (5).
Open repair of the descending thoracic aorta is associated with increased risk of neurological, cardiovascular and respiratory complications when compared with endovascular repair (8,9). There is a paucity of evidence to compare endovascular and open repair following vascular injury due to spinal surgery, but endovascular repair is a safe alternative to open thoracotomy for the repair of thoracic intercostal arteries due to trauma or iatrogenic injury avoiding the complications of thoracotomy, with one group reporting a success rate of up to 87.5% (10). Another series reported transarterial embolisation to be successful in all 20 patients that were treated for iatrogenic lumbar artery injury (11). Both these management techniques, however, require repositioning and transfer of the patient to permit access and visualisation for intervention. This is unsafe in the presence of uncontrolled haemorrhage and destabilised spinal cord and nerves, and results in delay.
Popliteal access does not involve repositioning the patient, does not require exposure of the groin, and can lead to faster control of the bleeding vessel, and prevent inadvertent de-sterilisation of the spinal wound. It may be limited by concomitant peripheral arterial disease causing stenosis or occlusion of the popliteal or superficial femoral artery and is relatively inaccessible in the supine position. We recommend popliteal puncture in the P1 segment to reduce the impact from limited length delivery systems. Sizing of the graft diameter can be done acutely using preoperative CT scan imaging performed routinely for the neurosurgical procedure and minimal oversizing is necessary. Length of device required cannot be measured easily but can reasonably be assumed to be short, and related to the length of those vertebral bodies manipulated during surgery. Bony landmarks can be used for deployment once major vessels like the coeliac are demonstrated and protected, minimising the need for angiography, and reducing the risk of major organ vascular compromise. We believe this approach has a role in the repair of other vascular injuries during posterior spinal procedures and surgical teams should be aware that such a route of endovascular repair is a viable option given appropriate expertise and imaging. We are hopeful further case series utilising this approach may emerge following our description of this novel approach. This will help assess its safety and efficacy, as well as providing evidence to support prospective cohort studies in the future. End points investigated can potentially include time to control of haemorrhage, spinal cord injuries and other long-term outcomes, which is benchmarked directly against traditional practice involving wound closure and turning the patient for angiography.
Conclusions
Vascular injury during spinal surgery can result in catastrophic haemorrhage. We describe a novel vascular access for TEVAR placement via the popliteal artery, allowing deployment of the TEVAR graft in the prone position, leading to early control of haemorrhage without the need to reposition or transfer the patient. The surgical teams should be aware such an approach exists with local expertise, and can be used as a viable alternative to the traditional femoral surgical cutdown access.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-23-17/rc
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-23-17/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-23-17/coif). JH is a director of the not-for-profit fellowship exam preparation course which receives financial support from GORE®. JH has multiple honoraria over the last 36 months with GORE® for lectures, presentations and expert round tables. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Giotta Lucifero A, Gragnaniello C, Baldoncini M, et al. Rating the incidence of iatrogenic vascular injuries in thoracic and lumbar spine surgery as regards the approach: A PRISMA-based literature review. Eur Spine J 2021;30:3172-90. [Crossref] [PubMed]
- Fantini GA, Pappou IP, Girardi FP, et al. Major vascular injury during anterior lumbar spinal surgery: incidence, risk factors, and management. Spine (Phila Pa 1976) 2007;32:2751-8. [Crossref] [PubMed]
- Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. J Med Case Rep 2013;7:223. [Crossref] [PubMed]
- Kakkos SK, Shepard AD. Delayed presentation of aortic injury by pedicle screws: report of two cases and review of the literature. J Vasc Surg 2008;47:1074-82. [Crossref] [PubMed]
- Li Z, Guo L, Zhang P, et al. A Systematic Review of Perioperative Complications in en Bloc Resection for Spinal Tumors. Global Spine J 2023;13:812-22. [Crossref] [PubMed]
- Oskouian RJ Jr, Johnson JP. Vascular complications in anterior thoracolumbar spinal reconstruction. J Neurosurg 2002;96:1-5. [Crossref] [PubMed]
- Tomita K, Kawahara N, Murakami H, et al. Total en bloc spondylectomy for spinal tumors: improvement of the technique and its associated basic background. J Orthop Sci 2006;11:3-12. [Crossref] [PubMed]
- Cheng D, Martin J, Shennib H, et al. Endovascular aortic repair versus open surgical repair for descending thoracic aortic disease a systematic review and meta-analysis of comparative studies. J Am Coll Cardiol 2010;55:986-1001. [Crossref] [PubMed]
- Xie X, Shu X, Zhang W, et al. A Comparison of Clinical Outcomes of Endovascular Repair Versus Open Surgery for Ruptured Descending Thoracic Aorta. J Endovasc Ther 2022;29:307-18. [Crossref] [PubMed]
- Chemelli AP, Thauerer M, Wiedermann F, et al. Transcatheter arterial embolization for the management of iatrogenic and blunt traumatic intercostal artery injuries. J Vasc Surg 2009;49:1505-13. [Crossref] [PubMed]
- Liu L, Li N, Wang Q, et al. Iatrogenic Lumbar Artery Injury in Spine Surgery: A Literature Review. World Neurosurg 2019;122:266-71. [Crossref] [PubMed]


