
Drain blockage by topical vancomycin powder—a case report of a potentially catastrophic complication in spine surgery
Highlight box
Key findings
• Vancomycin powder applied topically within surgical sites may cause drain occlusion.
What is known and what is new?
• Vancomycin powder may fail to dissolve when prepared in an insufficient volume of solution.
• The potential for powder residue to cause drain obstruction has not been previously reported.
What is the implication, and what should change now?
• The use of larger calibre drains would prevent this potential complication from occurring.
• Topical vancomycin may also be applied as a solution or paste in order to prevent drain occlusion.
Introduction
There have been many studies supporting the use of topical vancomycin powder to reduce the incidence of surgical site infection (SSI). Studies on the application of topical vancomycin powder following spine surgery are plentiful and have concluded that the incidence of SSIs are significantly reduced (1). Other examples on the efficacy of vancomycin powder to reduce infection rate include over sternotomy wounds (2), craniotomy sites (3), and following joint replacement (4). Topical vancomycin is also commonly utilized following surgical debridement of infections, which is supported by preclinical studies (5) albeit with less clinical evidence. Regardless of context, the application of topical vancomycin powder has been widely regarded as safe. We report here for the first time an episode of surgical drain blockage by vancomycin powder following surgical drainage in a patient suffering from L4/5 spondylodiscitis and an accompanying L1–S1 epidural abscess. Drain blockage by antibiotic powder may have devastating consequences following spine surgery. We present this case in accordance with the CARE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-23-46/rc).
Case presentation
A 62-year-old female with good past health presented to the emergency department with a 1-week history of fever and chills. This was associated with low back pain radiating to below the right knee. She did not complain of any abdominal or urinary symptoms. Travel and contact history were unremarkable.
Upon physical examination, her body temperature was 39.3 ℃, whilst her blood pressure and pulse were stable. The chest was clear to auscultation and the abdomen was soft, without guarding or rigidity. There was no flank or back tenderness. Right lower limb power was full, and sensation to fine touch was intact across lower limb dermatomes. Per-rectal examination revealed normal tone, grip, and sensation.
Blood tests demonstrated elevation of white blood cells to 18.54×109/L (normal range, 3.89–9.93) with a neutrophil predominance of 14.22×109/L (normal range, 2.01–7.42). Renal and liver function revealed hyponatremia of 121 mmol/L (normal range, 136–148), hypokalemia of 3.2 mmol/L (normal range, 3.6–5.0), normal renal function [estimated glomerular filtration rate (eGFR) >90], raised total bilirubin to 49 µmol/L (normal range, 4–23), raised alkaline phosphatase to 398 U/L (normal range, 47–124), normal alanine aminotransferase (ALT) of 45 U/L, normal aspartate aminotransferase (AST) of 31 U/L, normal amylase of 32 U/L and normal lactate of 1.1 mmol/L. An X-ray of the lumbar spine demonstrated reduced L4/5 disc space height (Figure 1A). Abdominal and chest X-rays were unremarkable.
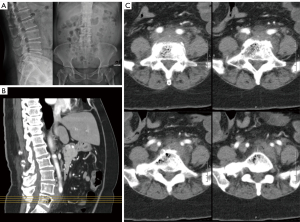
An urgent contrast computed tomography (CT) of the abdomen was performed due to suspicion of acute cholangitis. No abnormalities of the viscera were noted. However, intraosseous gas was seen within the L5 vertebra and L4/5 intervertebral disc (Figure 1B). There was associated retroperitoneal stranding and prominent lymph nodes.
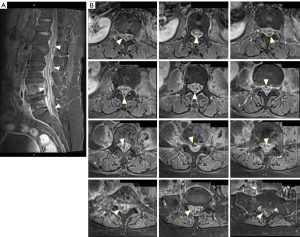
Due to the working diagnosis of L4/5 spondylodiscitis, a contrast lumbar spine magnetic resonance imaging (MRI) was arranged. On day 3 following admission, the patient developed weakness of the right tibialis anterior and right extensor hallucis weakness with a power of grade 3/5 according to the MRC scale. This was accompanied by increased back pain and a decrease in fine touch sensation from the right anterior thigh downwards.
MRI findings (Figure 2) were compatible with L4/5 spondylodiscitis and demonstrated T2 hyperintensity as well as contrast enhancement within the L4/5 disc space. Evidence of osteomyelitis was suggested over L4 and L5 vertebra from heterogeneous T1 hypointense/T2 hyperintense marrow signal changes. Multiple T1 hypointense, T2 hyperintense rim-enhancing epidural abscesses were noted ventral to the cauda equina at the level of the L5 vertebra, and posterior to the conus medullaris/cauda equina over L1–L3 and L5–S1 vertebral levels. Associated spinal stenosis and cerebrospinal fluid (CSF) effacement was noted at these levels.
The patient consented to emergent disc space and epidural abscess drainage in view of her neurological deterioration. She was anaesthetised, turned prone, and placed upon a Wilson frame. After skin preparation, the L1 to L5 lamina and facet joints were exposed on the right side. She received a right-sided hemilaminectomy over L1 to L5, and partial right facetectomy over L4/5, to respectively drain the dorsal epidural component and allow access to the L4/5 disc space and ventral epidural space. Exploration of the L4/5 disc space revealed infected disc material, which was thoroughly debrided. Copious irrigation with 6 L of normal saline was performed over the epidural space and L4/5 disc with the aid of an infant feeding tube. Hemostasis was achieved using bipolar cauterization and Floseal® hemostatic matrix. 1 gram of vancomycin powder was placed deep to the fascial layer, and tubing from a low pressure wound drain system (Exudrain®) was secured deep to the fascia. The fascia, dermis, and skin were closed in layers.
Immediately post-operatively, the patient reported improvement of her back pain and resolution of her right lower limb sensory complaints. Drain output, however, was minimal for the first 36 hours. The drain was shifted outwards by 2 cm with drainage monitored for a further 24 hours. This did not result in resumption of drainage output (Figure 3A). Inspection and disconnection of the distal drain tubing noted powder residue upon the drain lumen (Figure 3B), and complete obstruction at the narrow connection point between proximal (patient-side) and distal tubing (Figure 3C). An absence of drain output since surgery accompanied by evidence of solid powder residue indicated that early blockage occurred with vancomycin being the culprit. After exchange of the distal drain tubing which contained the occluded connection point, there was resumption of serosanguineous drain output of 30 mL over the first 24 hours, and 35 mLs over the next 24 hours. The drain was removed on post-operation day 5. Intra-operative specimen as well as blood cultures were positive for Bacteroides fragilis. Upon microbiology review the patient received IV moxifloxacin and metronidazole. She was transferred to the rehabilitation hospital on day 6 post surgery to receive physical therapy and to continue IV antibiotics. By week 3 post-op, her C-reactive protein (CRP) level had normalized, and right distal lower limb power improved to grade 4/5.

All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Verbal informed consent was obtained from the patient for publication of this case report and accompanying images.
Discussion
Meta-analysis on the use of topical vancomycin in spine surgery has concluded that it is effective in the prevention of SSI, especially against Gram-positive bacteria and methicillin-resistant Staphylococcus aureus (MRSA) (1,6). The bactericidal effect of intrawound vancomycin has similarly been consolidated in pre-clinical studies (7). From a biological standpoint, adverse effects from vancomycin wound application such as systemic absorption resulting in supratherapeutic drug concentrations, nephrotoxicity, and ototoxicity are exceedingly rare (8). There is some evidence that topical vancomycin inhibits bony healing, albeit not at weight-dosage equivalents that are of clinical relevance (9). Overall, topical application of vancomycin is considered to present minimal risk, with a rate of adverse events of just 0.3% (8).
To the best of our knowledge, this is the first reported incidence of drain blockage following antibiotic powder application. As a potential sequalae, hematoma formation may occur to cause neurological deterioration. This is a dreaded complication in spine surgery that has an estimated incidence of 0.10–0.86% (10). Drain malfunction following surgical debridement is also detrimental to bacterial clearance. Upon review, there were certain predisposing factors to drain blockage. Firstly, the low pressure wound drain system preferred at our center possessed a narrow choke point where proximal and distal limbs of the drain tubing docked with one another. The drain outer lumen diameter over this choke point was measured at 2.73±0.09 mm using a digital caliper, whilst the drain outer lumen diameter over the patient-side tubing prior to this choke point was 3.39±0.12 mm. Others prefer to use larger calibre drainage systems, with examples being the medium-sized Hemovac system (11) and the Jackson-Pratt drainage system (12). Secondly, the patient only received unilateral dissection of the lumbar spine and a total of 1 gram of vancomycin was placed within this small space. In the absence of significant blood volume, vancomycin powder would have failed to dissolve, and of relevance, precipitation of vancomycin has been described at concentrations 83.3 mg/mL and above when prepared in normal saline (13). This explained the presence of visible powder residue over the drain lumen and connection point. A limitation in our case was that we did not send the powder residue for formal microscopic or chemical analysis to confirm its composition.
This complication may be prevented by means of using a larger calibre drain, or via placement of vancomycin powder superficial to the fascia whilst the drain is placed subfascially. Another protective measure is to apply vancomycin in non-powder form. Total dissolution of vancomycin powder or formation of a paste prior to topical application has been described (2). Use of water as a solvent during preparation may be preferred since vancomycin has a lower solubility in normal saline. This is why vancomycin powder is first dissolved in water and further diluted in saline in preparation for IV infusion (14). Temporary clamping of the drain tubing often follows vancomycin powder application in joint arthroplasty to facilitate antibiotic dissolution and retention (15) but may be a hazard in spine surgery due to the potential for compression upon neural elements.
Conclusions
The application of vancomycin powder for the prevention of SSIs and treatment of infections requiring surgical debridement is evidence-based and prevalent. One should be aware of the physicochemical properties of vancomycin powder that affect dissolution and thereby potentially cause drain blockage. This is especially devastating in spine surgery when epidural hematoma formation may cause irreversible neurological sequalae.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The author has completed the CARE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-23-46/rc
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-23-46/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-23-46/coif). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Verbal informed consent was obtained from the patient for publication of this case report and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Luo H, Ren Y, Su Y, et al. Intraoperative vancomycin powder to reduce surgical site infections after posterior spine surgery: a systematic review and meta-analysis. EFORT Open Rev 2022;7:109-21. [Crossref] [PubMed]
- Donovan TJ, Sino S, Paraforos A, et al. Topical Vancomycin Reduces the Incidence of Deep Sternal Wound Complications After Sternotomy. Ann Thorac Surg 2022;114:511-8. [Crossref] [PubMed]
- Abdullah KG, Attiah MA, Olsen AS, et al. Reducing surgical site infections following craniotomy: examination of the use of topical vancomycin. J Neurosurg 2015;123:1600-4. [Crossref] [PubMed]
- Lawrie CM, Jo S, Barrack T, et al. Local delivery of tobramycin and vancomycin in primary total knee arthroplasty achieves minimum inhibitory concentrations for common bacteria causing acute prosthetic joint infection. Bone Joint J 2020;102-B:163-9. [Crossref] [PubMed]
- Wei J, Tong K, Zhou S, et al. Intra-wound vancomycin powder for the eradication of periprosthetic joint infection after debridement and implant exchange: experimental study in a rat model. BMC Microbiol 2021;21:333. [Crossref] [PubMed]
- Dodson V, Majmundar N, Swantic V, et al. The effect of prophylactic vancomycin powder on infections following spinal surgeries: a systematic review. Neurosurg Focus 2019;46:E11. [Crossref] [PubMed]
- Zebala LP, Chuntarapas T, Kelly MP, et al. Intrawound vancomycin powder eradicates surgical wound contamination: an in vivo rabbit study. J Bone Joint Surg Am 2014;96:46-51. [Crossref] [PubMed]
- Ghobrial GM, Cadotte DW, Williams K Jr, et al. Complications from the use of intrawound vancomycin in lumbar spinal surgery: a systematic review. Neurosurg Focus 2015;39:E11. [Crossref] [PubMed]
- Mendoza MC, Sonn KA, Kannan AS, et al. The effect of vancomycin powder on bone healing in a rat spinal rhBMP-2 model. J Neurosurg Spine 2016;25:147-53. [Crossref] [PubMed]
- Butler AJ, Mohile N, Phillips FM. Postoperative Spinal Hematoma and Seroma. J Am Acad Orthop Surg 2023;31:908-13. [Crossref] [PubMed]
- Blank J, Flynn JM, Bronson W, et al. The use of postoperative subcutaneous closed suction drainage after posterior spinal fusion in adolescents with idiopathic scoliosis. J Spinal Disord Tech 2003;16:508-12. [Crossref] [PubMed]
- Hughes SA, Ozgur BM, German M, et al. Prolonged Jackson-Pratt drainage in the management of lumbar cerebrospinal fluid leaks. Surg Neurol 2006;65:410-4, discussion 414-5. [Crossref] [PubMed]
- d'Huart É, Vigneron J, Charmillon A, et al. Physicochemical Stability of Vancomycin at High Concentrations in Polypropylene Syringes. Can J Hosp Pharm 2019;72:360-8.
- Masse M, Genay S, Martin Mena A, et al. Evaluation of the stability of vancomycin solutions at concentrations used in clinical services. Eur J Hosp Pharm 2020;27:e87-92.
- Amerstorfer F, Fischerauer S, Sadoghi P, et al. Superficial Vancomycin Coating of Bone Cement in Orthopedic Revision Surgery: A Safe Technique to Enhance Local Antibiotic Concentrations. J Arthroplasty 2017;32:1618-24. [Crossref] [PubMed]


