
Magnetically controlled growing rod treatment for early-onset scoliosis: analysis of 52 consecutive cases demonstrates improvement of coronal deformity
Highlight box
Key findings
• Magnetically controlled growing rods enables and maintains spinal deformity correction.
• The rate of unplanned surgeries did not differ significantly between single and dual rods.
• Thoracic height increased significantly in primary cases but not in conversion cases.
What is known and what is new?
• Magnetically controlled growing rods (MCGR) treatment allows spinal growth. This is known since earlier.
• There are no significant differences in complication rates or unplanned surgeries between the groups treated with single or dual rods. This is a new finding.
What is the implication, and what should change now?
• Both constructs of single and dual rods can be used in the future. MCGR is less useful in conversion surgery.
Introduction
Early-onset scoliosis (EOS) is defined as scoliosis with onset before 10 years of age. Patients with EOS have a significantly increased mortality rate compared with the general population if the scoliosis is left untreated (1).
Historically, growth friendly surgical management of EOS has been performed using implants that require frequent surgical lengthening procedures (2-4). Although facilitating curve correction and spinal growth, the inherent need for repeated surgical lengthening and high complication rates are major disadvantages (3,5-7).
Magnetically controlled growing rods (MCGR) have been developed recently to facilitate non-surgical lengthening, thus reducing the number of surgeries required for the management of EOS (8,9). Results regarding curve correction and spinal growth during MCGR treatment are encouraging (8-11). In addition, MCGR treatment is estimated to be cost neutral compared with other growth friendly surgical strategies (12-14). However, MCGR treatment is associated with a substantial risk of complications and unplanned surgeries, and concerns have been raised regarding the structural integrity of the MCGR implant (8,15-19). Most published studies include small numbers of patients, with heterogeneous samples and short follow-up times (8,10,15,20). The objective of this study was to report the radiographic results and complications in a consecutive series of patients treated with MCGR for EOS at two Swedish institutions. We present this article in accordance with the STROBE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-22-70/rc).
Methods
Study design
This consecutive series included all patients treated with MCGR for EOS at two Swedish institutions (Linköping University Hospital and Karolinska University Hospital) between December 2011 and September 2018. Patients were identified via local surgical records. The treatment for all patients was fully financed within the public health care system.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Swedish Ethics Review Authority (No. 2020-01473). The Swedish Ethics Review Authority confirmed that consent for inclusion and publication was not required in view of the retrospective nature of the study and that all procedures were part of the routine care.
Surgical technique and use of MCGR
First- and second-generation MAGEC rods (Nuvasive, San Diego, CA, USA) were used. The patients were analysed as a group but also divided into subgroups according to if they were treated with single or dual rods, primary surgically treated with MCGR or converted from previous Vertical Expandable Prosthetic Titanium Rib (VEPTR) treatment. The indications for conversion to MCGR from other surgical methods were failed previous surgical management or expected benefit from non-surgical lengthening procedures. Lengthening procedures were managed with outpatient visits, curve development and predicted growth pattern determined the frequency of lengthening procedures. Radiographs were used to monitor distraction of the MCGR after lengthening.
Analysis of the radiographic results and complications
Calibrated radiographs taken before, during, and after MCGR treatment were reviewed retrospectively by the first author of the study, using Sectra PACS IDS7 version 18.2 (Sectra AB, Linköping, Sweden). All measurements were crosschecked twice on calibrated radiographs by the first author of the study. The Cobb angle and thoracic kyphosis were measured using the Cobb method (21). The T1–T12 height and T1–S1 height were measured as described by Cheung et al. (22). For measurement of thoracic kyphosis, T1–T12 height, and T1–S1 height in cases with an abnormal number of thoracic vertebrae, the inferior endplate on the most distal thoracic vertebra (defined by the existence of at least one rib) was used as the distal measuring point. Lung height was measured in the frontal plane in a straight line from the lung apex to the apex of each hemidiaphragm. Growth of the T1–T12 and T1–S1 segments after MCGR implantation was calculated as the latest postoperative value minus the first postoperative value. The postoperative growth rate was calculated by dividing the growth value by the time elapsed between the first postoperative value and the latest postoperative value. The annual postoperative growth rate of the T1–T12 and T1–S1 segments was calculated in patients with a minimum postoperative radiographic follow-up of 1 year to avoid overestimation.
The patients’ medical records were reviewed for demographics and complications. All adverse events related to MCGR treatment that required any form of action, unplanned monitoring or led to patient harm were recorded as complications. For the subgroup analysis, the cohort was divided into primary versus conversion cases and single rods versus dual rods. Patients without previous surgical treatment of EOS were assigned to the primary group. Patients who had undergone previous surgical treatment of EOS were assigned to the conversion group. Index surgeries, surgeries solely for replacement of fully distracted MCGRs and scheduled definitive surgeries were labeled as planned. Revision procedures and definitive surgeries that were performed ahead of schedule due to complications were labeled as unplanned.
Statistical analysis
SPSS Statistics version 26 (IBM, Armonk, NY, USA) was used for statistical analysis. Before analysis, data were tested for normality using Z values of skewness and kurtosis, Shapiro-Wilk test, histograms, and Q-Q plots. One-way analysis of variance (ANOVA) with Tukey’s honest significant difference post hoc analysis and independent samples t-test (two-tailed) were used for statistical comparison. Pearson’s correlation coefficient was used to assess the relationship between radiographic follow-up time and T1–T12 height. If not otherwise specified, data presented as mean ± standard deviation. The significance level was set at 0.05.
Results
The demographics of the 52 patients included in the study and their respective EOS etiology are presented in Table 1. The mean age of MCGR implantation was 8.1 (range, 2.0–16.1) years, and during the mean follow-up time of 3.7 (range, 2.0–7.6) years, the overall mean number of MCGR lengthening was 13.7 (range, 0–46). Of the three deaths that occurred during ongoing MCGR treatment, one patient died from postoperative pneumonia during the initial postoperative period and is thus excluded from the postoperative analysis of the radiographic parameters; one patient died from ileus 1.8 years after MCGR implantation; one patient died from pneumonia 3.3 years after MCGR implantation (the last two deaths were unrelated to MCGR treatment and did not occur in close association with EOS surgery).
Table 1
| Patient demographics | Primary (n=32) | Conversion (n=20) | Single rod (n=28) | Dual rod (n=24) | Overall (n=52) |
|---|---|---|---|---|---|
| Age at MCGR implantation (years), mean (range)† | 7.4 (2.0–14.6) | 9.3 (5.0–16.1) | 8.2 (2.0–16.1) | 8.1 (4.9–11.9) | 8.1 (2.0–16.1) |
| Follow-up time (years), mean (range) | 3.8 (2.0–7.6) | 3.6 (2.0–6.8) | 3.6 (2.0–7.5) | 3.8 (2.0–7.6) | 3.7 (2.0–7.6) |
| Sex, n | |||||
| Female | 15 | 15 | 19 | 11 | 30 |
| Male | 17 | 5 | 9 | 13 | 22 |
| Etiology, n | |||||
| Neuromuscular | 15 | 7 | 7 | 15 | 22 |
| Congenital | 11 | 7 | 14 | 4 | 18 |
| Syndromic | 5 | 3 | 3 | 5 | 8 |
| Idiopathic | 1 | 3 | 4 | 0 | 4 |
| Previous surgical treatment of EOS, n | |||||
| None | 32 | 0 | 15 | 17 | 32 |
| VEPTR | 0 | 16 | 10 | 6 | 16 |
| TGR | 0 | 2 | 2 | 0 | 2 |
| Shilla | 0 | 1 | 0 | 1 | 1 |
| Single segment fusion | 0 | 1 | 1 | 0 | 1 |
| Type of MCGR-construct, n | |||||
| Single rod | 15 | 13 | – | – | 28 |
| Dual rod | 17 | 7 | – | – | 24 |
| Number of MCGR lengthening, mean (range) | 13.8 (0–46) | 13.5 (1–30) | 15.3 (3–46) | 11.8 (0–25) | 13.7 (0–46) |
| Number of surgeries | |||||
| Planned‡ | 45 | 33 | 45 | 33 | 78 |
| Unplanned | 20 | 16 | 24 | 12 | 36 |
| Total | 65 | 49 | 69 | 45 | 114 |
| Death during treatment period, n | 3 | 0 | 1 | 2 | 3 |
| Completed MCGR treatment, n | |||||
| Spinal fusion | 5 | 5 | 8 | 2 | 10 |
| MCGR removal§ | 1 | 2 | 3 | 0 | 3 |
| Conversion to VEPTR | 0 | 1 | 0 | 1 | 1 |
| Total | 6 | 8 | 11 | 3 | 14 |
Demographics of the 52 patients treated with MCGR. Patients divided into those treated with primary or conversion surgery, and single or dual rods. †, one congenital case with skeletal immaturity and expected residual spinal growth was converted from VEPTR to MCGR at age 16.1 years; ‡, index surgeries, surgeries solely for replacement of fully distracted MCGRs, and scheduled definitive operations; §, without spinal fusion. EOS, early-onset scoliosis; MCGR, magnetically controlled growing rod; TGR, traditional growing rod; VEPTR, vertical expandable prosthetic titanium rib.
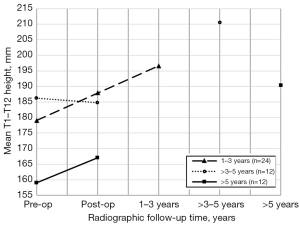
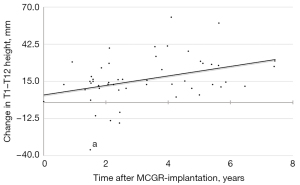
The development of the Cobb angle, T1–T12 height, and T1–S1 height are shown in Tables 2-4, respectively. Stratification based on radiographic follow-up time showed a statistically significant increase in T1–T12 height from postoperative to final follow-up in patients with a minimum radiographic follow-up time of 5 years (P=0.015; Figure 1). The mean postoperative increase in T1–T12 height in this subgroup was 23.4 mm. The increase in T1–T12 height was 4.2±8.3 mm/year in patients with 1–3 years of radiographic follow-up (n=24), 6.2±4.4 mm/year in patients with >3–5 years of radiographic follow-up (n=12), and 3.5±2.7 mm/year in patients with more than 5 years of radiographic follow-up (n=12). There was a significant positive relationship between radiographic follow-up time and development of T1–T12 height after MCGR implantation r=0.37, P=0.007; Figure 2). The mean T1–T12 height was significantly increased in the primary group and overall but not in the converted group alone.
Table 2
| Group of subjects | Preoperative (I), ° |
Postoperative (II), ° |
Final follow-up (III), ° | P value | |||
|---|---|---|---|---|---|---|---|
| ANOVA | I vs. II | I vs. III | II vs. III | ||||
| Primary (n=32) | 66±18 | 42±18 | 47±19 | <0.001 | <0.001 | <0.001 | ns |
| Conversion (n=20) | 55±14 | 41±12 | 45±16 | 0.008 | 0.007 | ns | ns |
| Single rod (n=28) | 60±18 | 41±15 | 42±19 | <0.001 | 0.001 | 0.001 | ns |
| Dual rod (n=24) | 64±17 | 42±16 | 51±16 | <0.001 | <0.001 | 0.023 | ns |
| Overall (n=52) | 62±17 | 42±16 | 46±18 | <0.001 | <0.001 | <0.001 | ns |
Values are presented as mean ± standard deviation. ANOVA, analysis of variance; ns, not statistically significant.
Table 3
| Group of subjects | Preoperative (I), mm | Postoperative (II), mm | Final follow-up (III), mm | Growth (mm/year)† |
P value | |||
|---|---|---|---|---|---|---|---|---|
| ANOVA | I vs. II | I vs. III | II vs. III | |||||
| Primary (n=32) | 174±30 | 180±29 | 199±30 | 5.1±7.2 | 0.005 | ns | 0.005 | 0.048 |
| Conversion (n=20) | 181±40 | 187±42 | 201±43 | 3.5±5.0 | ns | – | – | – |
| Single rod (n=28) | 173±40 | 183±41 | 200±39 | 4.0±5.0 | ns | – | – | – |
| Dual rod (n=24) | 181±27 | 183±27 | 199±31 | 5.1±8.1 | ns | – | – | – |
| Overall (n=52) | 177±34 | 183±35 | 199±35 | 4.5±6.5 | 0.004 | ns | 0.004 | 0.047 |
Values are presented as mean ± standard deviation. †, growth after magnetically controlled growing rod implantation. ANOVA, analysis of variance; ns, not statistically significant.
Table 4
| Group of subjects | Preoperative (I), mm |
Postoperative (II), mm | Final follow-up (III), mm | Growth (mm/year)† |
P value | |||
|---|---|---|---|---|---|---|---|---|
| ANOVA | I vs. II | I vs. III | II vs. III | |||||
| Primary (n=32) | 284±47 | 301±45 | 324±46 | 7.0±6.7 | 0.004 | ns | 0.003 | ns |
| Conversion (n=20) | 299±60 | 315±60 | 330±62 | 4.6±8.3 | ns | – | – | – |
| Single rod (n=28) | 293±63 | 310±61 | 330±65 | 5.4±6.9 | ns | – | – | – |
| Dual rod (n=24) | 286±37 | 301±38 | 321±30 | 7.2±7.8 | 0.005 | ns | 0.004 | ns |
| Overall (n=52) | 290±53 | 306±51 | 326±52 | 6.2±7.3 | 0.003 | ns | 0.002 | ns |
Values are presented as mean ± standard deviation. †, growth after magnetically controlled growing rod implantation. ANOVA, analysis of variance; ns, not statistically significant.
Mean overall thoracic kyphosis changed from 41° (19°) preoperatively to 32° (14°) postoperatively to 39° (17°) at final follow-up (P=0.018). The mean overall left lung height changed from 130 mm (35 mm) preoperatively to 129 mm (31 mm) postoperatively to 146 mm (35 mm) at final follow-up (P=0.011). Mean overall right lung height changed from 123 mm (35 mm) preoperatively to 123 mm (32 mm) postoperatively to 140 mm (36 mm) at final follow-up (P=0.019). The increase in lung height from postoperative to final follow-up was statistically significant for both left lung height (P=0.019) and right lung height (P=0.036).
Details of the complications are shown in Table 5. The overall complication rate mean (range) was 1.4 (0–4). There were no significant differences in complication rates between subgroups. Twenty-nine complications (in 22 patients) required 36 unplanned surgeries. There were 24 unplanned surgeries in the single-rod group and 12 unplanned surgeries in the dual-rod group (P=0.154). In one case, spinal cord damage was suspected at the index surgery. The MCGR implant was immediately removed to facilitate magnetic resonance imaging (MRI) of the spinal cord. No neurologic injuries were identified on MRI or postoperative neurologic examinations, and MCGR was successfully implanted during a second index operation.
Table 5
| Primary (n=32) | Conversion (n=20) | Single rod (n=28) | Dual rod (n=24) | Overall (n=52) | |
|---|---|---|---|---|---|
| Distribution of complications, n | |||||
| 0 complications | 11 | 6 | 10 | 7 | 17 |
| 1 complication | 6 | 6 | 5 | 7 | 12 |
| >1 complication | 15 | 8 | 13 | 10 | 23 |
| Complications requiring unplanned surgery, n | 16 | 13 | 19 | 10 | 29 |
| Implant complications, n | |||||
| Failure of proximal foundation | 13 | 7 | 10 | 10 | 20 |
| Failure of distal foundation | 5 | 5 | 6 | 4 | 10 |
| Rod fracture | 3 | 1 | 4 | 0 | 4 |
| Failure to distract | 1 | 2 | 1 | 2 | 3 |
| Actuator pin fracture | 1 | 0 | 1 | 0 | 1 |
| Surgical site infection, n | |||||
| Superficial | 2 | 0 | 2 | 0 | 2 |
| Deep | 1 | 1 | 2 | 0 | 2 |
| Surgical and intraoperative complications, n | |||||
| Total MEP loss† | 1 | 1 | 0 | 2 | 2 |
| Dural tear | 0 | 1 | 1 | 0 | 1 |
| Damage to intrathecal catheter‡ | 0 | 1 | 0 | 1 | 1 |
| Pain during MCGR lengthening, n | 0 | 3 | 2 | 1 | 3 |
| Skin or soft tissue complications§, n | 4 | 2 | 4 | 2 | 6 |
| Medical or anesthesiology complications¶, n | 11 | 4 | 6 | 9 | 15 |
| Total number of complications, n | 42 | 28 | 39 | 31 | 70 |
| Number of complications, mean (range) | 1.3 (0–3) | 1.4 (0–4) | 1.4 (0–4) | 1.3 (0–3) | 1.4 (0–4) |
| P value# | 0.80 | 0.76 | |||
†, no permanent neurologic sequelae; ‡, catheter for an intrathecal baclofen pump was inadvertently cut at MCGR implantation, successfully repaired during the same surgical session; §, two cases of implant prominence (revision required), 2 pressure wounds, 1 slow-healing wound (>6 months), and 1 wound dehiscence (revision required); ¶, 9 postoperative infections (6 airway, 3 urinary tract), 2 dental injuries at intubation, 2 drug side effects (exanthematous skin rash and hepatitis), 1 laryngeal edema at extubation, and 1 jugular vein thrombosis caused by a central venous catheter; #, independent samples t-test (two-tailed) for tests of number of complications. MCGR, magnetically controlled growing rod; MEP, motor evoked potential.
Discussion
The main finding of this study was a significant correction of the Cobb angle from preoperative to final follow-up on a group level. This correlates well with other studies of MCGR treatment (8,10,15,20). However, the Cobb angle correction was non-significant among conversion cases, an expected finding also consistent with previous reports (8,23-26). Conversion cases have undergone previous surgical treatment, therefore their potential for further correction of the Cobb angle is likely diminished compared with primary cases. Importantly, the mean Cobb angles at final follow-up were almost identical for primary and conversion cases, with a difference of only 2° between the groups. Cobb angle correction was maintained after index surgery overall and on a subgroup level.
There was a statistically significant increase in T1–T12 height and T1–S1 height from preoperative to final follow-up. Correction of deformity at the initial implantation accounts for some gain in spinal height, however, and the aim of using growth friendly instrumentation is to facilitate spinal growth specifically in the period from implantation to final fusion. Previously published results regarding the growth rate of the T1–T12 segment are highly variable (from 1.5 to 13.2 mm/year) (9,16,22,25,27-29). The postoperative growth rate of the T1–T12 segment (4.5 mm/year) in our study is comparable with results reported by Lampe et al. (16) (4.4 mm/year) in a study with follow-up time similar to ours. Compared with healthy children, the growth rate of the T1–T12 and T1–S1 segments after MCGR implantation was reduced in our population (30). The mean T1–T12 height was significantly increased in the primary group and overall but not in the converted group alone. This can be due to the fact that the converted subgroup was in mean age two years older than the primary group. Another clinical observation that can be taken into consideration is the stiffening of the spine with repeated surgeries.
There was a positive correlation between radiographic follow-up time and T1–T12 height, suggesting that longer treatment time yields a greater increase in spinal height (Figure 2). Stratification revealed a statistically significant postoperative increase in T1–T12 height after 5 years of MCGR treatment (P=0.015; Figure 1). MCGR treatment evidently facilitates spinal growth. However, the time required to achieve sufficient spinal growth might be longer than previously thought. Although the above stratification is subject to selection bias, it does underline the importance of selecting patients with residual spinal growth for MCGR treatment and achieving an adequate duration of treatment. In our opinion, it is essential to identify predictors for adverse outcomes to increase treatment time and minimize the risk for early fusion or conversion to another growth friendly surgical strategy.
Compared with a systematic review of complications during MCGR treatment published by Thakar et al. (15), our overall complication rate (1.4 complications per patient) is high. However, the average follow-up time in their systematic review (2.5 years) is shorter than ours (mean 3.7 years). Furthermore, we have applied a broad definition of what is considered a complication, which has led to the inclusion of several minor complications. In addition, we have included medical complications and complications related to anesthesia (21% of our complications). Examples of minor complications in this cohort include two dental injuries at intubation, three occasions of pain at MCGR lengthening, and one exanthematous skin rash from intravenous β-lactam antibiotics.
Compared with studies with similar follow-up times, our rate of non-medical complications (55 complications, 1.1 complication per patient) is high (8,16-18). Several of our non-medical complications did not require unplanned revision surgery, and our rate of unplanned surgeries (69%, 36 unplanned surgeries in 52 patients) is lower than that in several of the other studies (16,18). In addition, a comparatively low proportion of the patients in our population (42%) underwent one or more unplanned surgeries (8,17,18). Complications such as foundation failures, rod fractures, skin or soft tissue problems, and wound infections are common among other growth friendly surgical techniques, and not unique to MCGR implants (3,5-7,31,32). In this population, the most frequent implant complication was failure of the proximal foundation. The most common proximal foundation used was rib hooks, which unilaterally migrated through or partially dislocated from the ribs in 12 patients. The current MCGR guidelines suggest removal after 2 years. However, this has not been implemented in this cohort treated before the new guidelines.
We present a large cohort of patients treated with single rods. Several previous publications do not recommend the use of single-rod constructions, mainly from a safety standpoint (15,33,34). From our results, it could be argued that, in a subset of patients with EOS, single rods might be as efficient and safe as dual rods. It is our clinical experience that congenital cases, which often have short and less flexible curves, are more suited to treatment with single rods. However, the design of this study does not allow for definite conclusions on this matter. In addition, our single- and dual-rod groups were not fully comparable, mainly regarding the etiology of EOS. The single-rod group consisted mainly of congenital cases, whereas the dual-rod group consisted mainly of neuromuscular cases. On a group level, the radiographic results were comparable between single and dual rods, but the Cobb angle correction was not as well maintained among dual-rod cases after the index surgery. This might be attributable to the high proportion of neuromuscular and syndromic cases of EOS in the dual-rod group. These cases tend to have a relatively flexible spinal deformity that is more prone to collapsing during treatment. Regarding safety, there was no statistically significant difference in the total number of complications between single and dual rods. Furthermore, the proportion of implant complications was comparable between single and dual rods (56% vs. 52%). The number of unplanned surgeries was markedly higher for single-rod cases, but the difference in unplanned surgeries did not reach statistical significance.
Limitations of this study include the retrospective design, the heterogeneity of the population, the fact that all radiographic measurements were performed by a single observer and the fact that complications were only mentioned but not further classified. Strengths include the consecutive sampling of all patients treated with MCGR from two different centers and the thorough recording of complications. A larger sample size will be of value in the future.
Conclusions
In this cohort, MCGR treatment enabled and maintained correction of EOS. Statistically significant growth of the T1–T12 segment was achieved after a minimum of 2 years follow-up. Deformity correction and spinal growth were comparable between cases treated with single versus dual rods. MCGR treatment for EOS carries a high risk of complications and unplanned surgeries. The rates of complications and unplanned surgeries did not differ significantly between single and dual rods but the etiology for EOS varied between the groups.
Acknowledgments
Funding: Funds were received from the Swedish Research Council (Dnr 2017-01639) (Grant holder, P Gerdhem). P Gerdhem was supported by Region Stockholm (clinical research appointment) and CIMED, Karolinska Institutet. The funding sources had no role in the study design, analyses, and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-22-70/rc
Data Sharing Statement: Available at https://jss.amegroups.com/article/view/10.21037/jss-22-70/dss
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-22-70/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-22-70/coif). FE reports that he was employed by Region Östergötland for clinical medical internship. As part of the employment (internship), 6 months were allocated for research work and 18 months for clinical work. Total duration of the employment was 24 months. The support from Region Östergötland consisted of regular monthly salaries during the employment. No additional support or funding was received. FE is now employed by Region Östergötland for clinical medical residency. PG reports that grants were received from the Swedish Research Council (Dnr 2017-01639) (payment to The Karolinska Institute), Region Stockholm (payment to The Karolinska University Hospital) and CIMED, Karolinska Institutet (payment to The Karolinska Institute). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Swedish Ethics Review Authority (Ref. 2020-01473). The Swedish Ethics Review Authority confirmed that consent for inclusion and publication was not required in view of the retrospective nature of the study and that all procedures were part of the routine care.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pehrsson K, Larsson S, Oden A, et al. Long-term follow-up of patients with untreated scoliosis. A study of mortality, causes of death, and symptoms. Spine (Phila Pa 1976) 1992;17:1091-6. [Crossref] [PubMed]
- Moe JH, Kharrat K, Winter RB, et al. Harrington instrumentation without fusion plus external orthotic support for the treatment of difficult curvature problems in young children. Clin Orthop Relat Res 1984;35-45.
- Akbarnia BA, Marks DS, Boachie-Adjei O, et al. Dual growing rod technique for the treatment of progressive early-onset scoliosis: a multicenter study. Spine (Phila Pa 1976) 2005;30:S46-57. [Crossref] [PubMed]
- Campbell RM Jr, Smith MD, Hell-Vocke AK. Expansion thoracoplasty: the surgical technique of opening-wedge thoracostomy. Surgical technique. J Bone Joint Surg Am 2004;86-A:51-64.
- Sankar WN, Acevedo DC, Skaggs DL. Comparison of complications among growing spinal implants. Spine (Phila Pa 1976) 2010;35:2091-6. [Crossref] [PubMed]
- Campbell RM Jr, Smith MD, Mayes TC, et al. The effect of opening wedge thoracostomy on thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am 2004;86:1659-74. [Crossref] [PubMed]
- Bess S, Akbarnia BA, Thompson GH, et al. Complications of growing-rod treatment for early-onset scoliosis: analysis of one hundred and forty patients. J Bone Joint Surg Am 2010;92:2533-43. [Crossref] [PubMed]
- Subramanian T, Ahmad A, Mardare DM, et al. A six-year observational study of 31 children with early-onset scoliosis treated using magnetically controlled growing rods with a minimum follow-up of two years. Bone Joint J 2018;100-B:1187-200. [Crossref] [PubMed]
- Akbarnia BA, Pawelek JB, Cheung KM, et al. Traditional Growing Rods Versus Magnetically Controlled Growing Rods for the Surgical Treatment of Early-Onset Scoliosis: A Case-Matched 2-Year Study. Spine Deform 2014;2:493-7. [Crossref] [PubMed]
- Akbarnia BA, Cheung K, Noordeen H, et al. Next generation of growth-sparing techniques: preliminary clinical results of a magnetically controlled growing rod in 14 patients with early-onset scoliosis. Spine (Phila Pa 1976) 2013;38:665-70. [Crossref] [PubMed]
- Cheung JPY, Yiu K, Kwan K, et al. Mean 6-Year Follow-up of Magnetically Controlled Growing Rod Patients With Early Onset Scoliosis: A Glimpse of What Happens to Graduates. Neurosurgery 2019;84:1112-23. [Crossref] [PubMed]
- Polly DW Jr, Ackerman SJ, Schneider K, et al. Cost analysis of magnetically controlled growing rods compared with traditional growing rods for early-onset scoliosis in the US: an integrated health care delivery system perspective. Clinicoecon Outcomes Res 2016;8:457-65. [Crossref] [PubMed]
- Charroin C, Abelin-Genevois K, Cunin V, et al. Direct costs associated with the management of progressive early onset scoliosis: estimations based on gold standard technique or with magnetically controlled growing rods. Orthop Traumatol Surg Res 2014;100:469-74. [Crossref] [PubMed]
- Rolton D, Richards J, Nnadi C. Magnetic controlled growth rods versus conventional growing rod systems in the treatment of early onset scoliosis: a cost comparison. Eur Spine J 2015;24:1457-61. [Crossref] [PubMed]
- Thakar C, Kieser DC, Mardare M, et al. Systematic review of the complications associated with magnetically controlled growing rods for the treatment of early onset scoliosis. Eur Spine J 2018;27:2062-71. [Crossref] [PubMed]
- Lampe LP, Schulze Bövingloh A, Gosheger G, et al. Magnetically Controlled Growing Rods in Treatment of Early-Onset Scoliosis: A Single Center Study With a Minimum of 2-Year-Follow up and Preliminary Results After Converting Surgery. Spine (Phila Pa 1976) 2019;44:1201-10. [Crossref] [PubMed]
- Kwan KYH, Alanay A, Yazici M, et al. Unplanned Reoperations in Magnetically Controlled Growing Rod Surgery for Early Onset Scoliosis With a Minimum of Two-Year Follow-Up. Spine (Phila Pa 1976) 2017;42:E1410-4.
- Teoh KH, Winson DM, James SH, et al. Do magnetic growing rods have lower complication rates compared with conventional growing rods? Spine J 2016;16:S40-4.
- Rushton PRP, Smith SL, Kandemir G, et al. Spinal Lengthening With Magnetically Controlled Growing Rods: Data From the Largest Series of Explanted Devices. Spine (Phila Pa 1976) 2020;45:170-6. [Crossref] [PubMed]
- Ridderbusch K, Rupprecht M, Kunkel P, et al. Preliminary Results of Magnetically Controlled Growing Rods for Early Onset Scoliosis. J Pediatr Orthop 2017;37:e575-80.
- Cobb JR. Outlines for the study of scoliosis. In: American Academy of Orthopedic Surgeons, ed. Instructional course lectures. Ann Arbor, MI: JW Edwards; 1948.
- Cheung KM, Cheung JP, Samartzis D, et al. Magnetically controlled growing rods for severe spinal curvature in young children: a prospective case series. Lancet 2012;379:1967-74. [Crossref] [PubMed]
- Thompson W, Thakar C, Rolton DJ, et al. The use of magnetically-controlled growing rods to treat children with early-onset scoliosis: early radiological results in 19 children. Bone Joint J 2016;98-B:1240-7. [Crossref] [PubMed]
- Hosseini P, Pawelek J, Mundis GM, et al. Magnetically controlled Growing Rods for Early-onset Scoliosis: A Multicenter Study of 23 Cases With Minimum 2 years Follow-up. Spine (Phila Pa 1976) 2016;41:1456-62. [Crossref] [PubMed]
- Keskinen H, Helenius I, Nnadi C, et al. Preliminary comparison of primary and conversion surgery with magnetically controlled growing rods in children with early onset scoliosis. Eur Spine J 2016;25:3294-300. [Crossref] [PubMed]
- Studer D, Heidt C, Büchler P, et al. Treatment of early onset spinal deformities with magnetically controlled growing rods: a single centre experience of 30 cases. J Child Orthop 2019;13:196-205. [Crossref] [PubMed]
- Dahl B, Dragsted C, Ohrt-Nissen S, et al. Use of a distraction-to-stall lengthening procedure in magnetically controlled growing rods: A single-center cohort study. J Orthop Surg (Hong Kong) 2018;26:2309499018779833. [Crossref] [PubMed]
- Lebon J, Batailler C, Wargny M, et al. Magnetically controlled growing rod in early onset scoliosis: a 30-case multicenter study. Eur Spine J 2017;26:1567-76. [Crossref] [PubMed]
- Heydar AM, Şirazi S, Bezer M. Magnetic Controlled Growing Rods as a Treatment of Early Onset Scoliosis: Early Results With Two Patients. Spine (Phila Pa 1976) 2016;41:E1336-42. Erratum in: Spine (Phila Pa 1976) 2017;42:E566.
- Dimeglio A, Canavese F. The growing spine: how spinal deformities influence normal spine and thoracic cage growth. Eur Spine J 2012;21:64-70. [Crossref] [PubMed]
- Garg S, LaGreca J, St Hilaire T, et al. Wound complications of vertical expandable prosthetic titanium rib incisions. Spine (Phila Pa 1976) 2014;39:E777-81. [Crossref] [PubMed]
- McCarthy RE, McCullough FL. Shilla Growth Guidance for Early-Onset Scoliosis: Results After a Minimum of Five Years of Follow-up. J Bone Joint Surg Am 2015;97:1578-84. [Crossref] [PubMed]
- Teoh KH, Winson DM, James SH, et al. Magnetic controlled growing rods for early-onset scoliosis: a 4-year follow-up. Spine J 2016;16:S34-9. [Crossref] [PubMed]
- Abdelaal A, Munigangaiah S, Trivedi J, et al. Magnetically controlled growing rods in the treatment of early onset scoliosis: a single centre experience of 44 patients with mean follow-up of 4.1 years. Bone Jt Open 2020;1:405-14. [Crossref] [PubMed]


