
Radical decompression without fusion for L5 radiculopathy due to foraminal stenosis
Highlight box
Key findings
• Our surgical technique, which includes unilateral removal of the pars interarticularis, does not compromise segmental stability at L5–S1.
What is known and what is new?
• The L5–S1 segment has different anatomical features.
• We exhaustively assessed surgical outcomes after our surgical technique, limiting the materials to patients with lumbar foraminal stenosis at the L5–S1 segment, and obtained a good surgical outcome.
What is the implication, and what should change now?
• For lumbar foraminal stenosis at L5–S1, decompression with adequate bony resection is effective. Our surgical technique can be one of the choices.
Introduction
Lumbar foraminal stenosis (LFS) causes radiculopathy with leg pain and/or muscle weakness. Surgical treatment is indicated in cases where conservative treatment fails to relieve symptoms. Various types of surgeries have been reported (1-6), including simple decompression by lateral fenestration (1), microendoscopic decompression (6), and posterior lumbar interbody fusion (PLIF) (5). However, each surgical procedure has its advantages and disadvantages. For example, microendoscopic decompression is a minimally invasive and technically demanding procedure (6), whereas PLIF is a common procedure used to treat this pathology; however, it carries a potential risk of implant-related complications (7,8).
The highest incidence of LFS occurs in the L5–S1 segment (9). This segment differs from others in two aspects. First, the L5 vertebra is anchored by the iliolumbar ligament (10); thus, this spinal segment may be more stable than the other segments. Second, in addition to intraforaminal stenosis, extraforaminal stenosis occurs more frequently here than at other spinal levels owing to the specific anatomical features of the lumbosacral junction (11). Diagnosing extraforaminal stenosis at the L5–S1 segment via imaging is reportedly difficult (12); therefore, this stenosis is occasionally overlooked, causing failed back surgery syndrome (13). Due to the anatomical features and difficulty of diagnosing LFS at L5–S1 by imaging, surgical outcomes for L5–S1 LFS should be assessed separately from those of the other lumbar spinal segments. However, to the best of our knowledge, few previous reports have exhaustively assessed surgical outcomes after decompression surgery, limiting the materials to patients with LFS at the L5–S1 segment.
We routinely decompress the L5 nerve root throughout its course, including the extraforaminal regions. Our surgical technique includes both the unilateral removal of the pars interarticularis, as reported by Tender et al. (4), and unilateral L5–S1 lateral fenestration, as reported by Kunogi et al. (1). We designated this combined procedure as “radical decompression”. Instrumented spinal fusion was not performed. Since postoperative segmental instability and insufficient decompression are risk factors associated with unsatisfactory outcomes of decompression surgery for LFS (2,3,14,15), we investigated instability at the L5–S1 segment and neurological improvement after “radical decompression” of the L5 nerve root. We present this article in accordance with the STROBE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-23-62/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Board of Tohoku Central Hospital (No. 107-1) and informed consent was taken from all the patients.
Patients
This prospective study enrolled patients with unilateral L5 radiculopathy due to LFS at L5–S1 who underwent “radical decompression surgery”. L5 radiculopathy was diagnosed based on symptomatology and the findings of a neurological examination performed by experienced spinal surgeons. The diagnosis of LFS was confirmed using three-dimensional (3D) magnetic resonance imaging (MRI), in which continuous oblique coronal, sagittal, and axial slices were assessed. The following five criteria were used for the imaging diagnosis of LFS: (I) obliteration of the perineural fat surrounding the L5 nerve root (16); (II) transverse path of the nerve root (12); (III) obscurity of the dorsal root ganglion (12); (IV) spinal nerve indentation (12); and (V) nerve swelling (12). Selective nerve root block of the L5 nerve root was performed to confirm the diagnosis. The surgical indications were as follows: (I) unilateral L5 radiculopathy due to L5–S1 foraminal stenosis; (II) severe muscle weakness or intolerable leg pain that was resistant to conservative treatments; (III) absence of spondylolysis; and (IV) selective nerve root block relieved the pain by ≥70%. All patients who met these criteria and underwent surgery using the radical decompression technique between September 2013 and August 2014 were enrolled and evaluated two years after surgery.
“Radical decompression” procedure
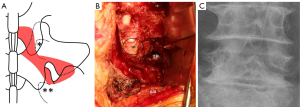
Under general anesthesia, the patient was placed in a prone position. The spinous processes of either L4 or L5 were marked with a K-wire and confirmed on frontal and lateral plain radiographs. A 7–10 cm midline incision was made from the lower part of the L3 spinous process to the upper part of the sacral spinous process, and the L4 and L5 laminae were exposed. Conventional unilateral fenestration of L4–L5 was performed using a high-speed air drill (17), followed by resection of the pars interarticularis (4) and the tip of the superior articular process of S1. The ligamentum flavum was removed, and the L5 nerve root and medial margin of the L5 pedicle were identified. The bony resection at the pars interarticularis was widened as the L5–S1 disc was detected at the caudal area of the L5 nerve root. Then, using the Wiltse approach with the same midline skin incision (18), an intermuscular plane between the longissimus and multifidus muscles was dissected to expose the extraforaminal region, including the L5 transverse process and sacral ala. The caudal portion of the L5 transverse process and sacral ala was partially resected to release nerve root impingement in the region. Finally, the lateral fenestration was connected to the parsectomy. The ligamentous or synovial-like tissue that compressed the nerve root was removed, and decompression of the L5 nerve root was confirmed at its entrance into the retroperitoneal space. Discectomy was performed if the disc bulging or herniation compressed the nerve root. Figure 1 shows the area of the bony resection (Figure 1A), an intraoperative photograph (Figure 1B), and a postoperative radiograph (Figure 1C).
Clinical assessments
Leg pain was assessed using the Visual Analogue Scale (VAS) preoperatively and two weeks postoperatively. The Japanese Orthopaedic Association (JOA) score (29 possible points) was obtained preoperatively and 2 years postoperatively. The JOA score recovery rate was calculated as (postoperative JOA score – preoperative JOA score)/(29 – preoperative JOA score) × 100 (%) (19). The results of the JOA score evaluation were classified into four groups according to the recovery rate: (I) poor: <25%; (II) fair: ≥25% to <50%; (III) good: ≥50% to <75%; and (IV) excellent: ≥75%. In addition, the JOA Back Pain Evaluation Questionnaire (JOABPEQ) (20) was administered preoperatively and at 2 years postoperatively to assess lower back pain. All five domains of the JOABPEQ were assessed. Treatment was considered effective when either of the following criteria were satisfied: (I) the postoperative score increased by ≥20 points compared to the preoperative score; or (II) the postoperative score was ≥90 points in patients with a preoperative score <90. Patients with preoperative and postoperative scores ≥90 were excluded from the evaluation according to the guidelines (20). The effectiveness rate of surgery was calculated in each domain using the following formula (20): (number of patients whose surgeries were considered effective)/[(total number of patients) - (number of patients whose pre- and postoperative scores were ≥90 points)].
Radiological measurements
Frontal and lateral radiographs in the neutral standing position, flexion-extension lateral radiographs, and MRI scans were obtained preoperatively and two years postoperatively. The following imaging parameters were measured at the L5–S1 segment (Figure 2): lateral translation (mm, Figure 2A), sagittal translation (mm, Figure 2B), difference in sagittal translation (DST) between flexion and extension (mm, Figure 2C), disc wedging angle (degrees, Figure 2D), lordotic angle (degrees, Figure 2E), difference in lordotic angle (DLA) between flexion and extension (degrees, Figure 2F), and disc height (mm, Figure 2G). Lateral translation was defined as the distance between the two vertical lines passing through the midpoints of the L5 and S1 pedicles (Figure 2A). Sagittal translation was defined as the distance between the posterosuperior margin of the sacrum and the line perpendicular to the sacral endplate passing through the posteroinferior margin of L5 in the neutral lateral view (Figure 2B). DST was calculated as (sagittal translation in flexion) – (sagittal translation in extension) (Figure 2C). The disc wedging angle was calculated as the lower endplate of L5 and the endplate of S1 in the frontal view (Figure 2D). The lordotic angle was defined as the angle between the lower endplates of L5 and the endplate of S1 on a neutral lateral view (Figure 2E). The DLA was calculated as (lordotic angle in extension) – (lordotic angle in flexion) (Figure 2F). Disc height was measured using midsagittal MRI because it could not be accurately measured on radiographs of patients with local scoliosis at L5–S1. The disc height was defined as the distance between the midpoints of the L5 lower endplate and S1 endplate (Figure 2G). All imaging parameters were measured using workstation software (EV Insite R, PSP Corporation, Tokyo, Japan). To assess the reliability of the measurements, radiological parameters were measured by two spine surgeons, and ten randomly selected patients were measured twice by one spine surgeon. Interclass correlation coefficients {ICC [2, 1]} for intraobserver and interobserver errors were obtained using SPSS software version 24 (IBM Corp., Armonk, NY, USA).

Statistical analyses
Pre- and postoperative clinical and radiological data were compared using paired t-tests. In addition, the patients were classified into a disc group (Group D) and a non-disc group (Group ND) according to whether a discectomy was performed intraoperatively. Changes in each parameter before and after surgery were compared between the groups. All continuous variables are expressed as the mean ± standard deviation, and P<0.05 was considered significant.
Results
In total, 29 patients were enrolled, but one patient died of cancer fifteen months after surgery; therefore, 28 patients were followed up for >2 years and were included in this study. Among them, fifteen were male, and thirteen were female. The mean age at surgery was 68 years (range, 51–81 years), and the mean duration of preoperative symptoms was 13 months (range, 0.5–60 months). There was one case of degenerative spondylolisthesis. The operative duration and the intraoperative blood loss were 149±31 minutes and 58±23 g, respectively. No intraoperative dural tears or postoperative surgical site infections were observed. Discectomy was performed in thirteen patients; these 13 cases were classified as Group D, and the other fifteen cases were classified as Group ND.
Clinical assessments
Preoperative radicular pain significantly improved 2 weeks after surgery. The preoperative leg pain VAS was 5.5±2.6 cm (range, 0–10 cm), which was significantly decreased by 0.7±1.0 cm (range, 0–3.1 cm) 2 weeks postoperatively (P<0.0001). The JOA score improved in all patients; the pre- to postoperative JOA scores significantly increased from 14.5±3.2 (range, 8–21) to 24.3±3.3 (range, 18–29), respectively (P<0.01). The average recovery rate was 66.9%±23.1% (range, 12.5–100%). The JOA score evaluation were excellent in 10 patients (36%), good in 13 (46%), fair in 3 (11%), and poor in 2 (7%).
All domains of the JOABPEQ improved 2 years postoperatively (P<0.05). The effectiveness rates in each domain were 76.9% (20/26 patients) for pain-related disorders, 52.0% (13/25 patients) for lumbar spine dysfunction, 85.7% (24/28 patients) for gait disturbance, 64.3% (18/28 patients) for social dysfunction, and 39.3% (11/28 patients) for psychological disorders. None of the patients underwent revision surgery. The preoperative and postoperative clinical data are presented in Table 1.
Table 1
| Clinical assessments | Preoperative | Postoperative | Average of difference (95% CI) | P |
|---|---|---|---|---|
| JOA score | 14.5±3.2 | 24.3±3.3 | 9.8 (8.3, 11.2) | <0.0001 |
| JOABPEQ | ||||
| Pain-related disorders | 40.3±27.0 | 76.5±29.1 | 36.2 (23.9, 48.5) | <0.0001 |
| Lumbar dysfunction | 63.9±27.8 | 77.9±20.1 | 14.0 (1.4, 26.5) | 0.03 |
| Gait disturbance | 28.0±21.4 | 77.3±27.4 | 49.3 (5.6, 60.8) | <0.0001 |
| Social life dysfunction | 39.4±20.3 | 69.3±21.4 | 29.9 (18.9, 40.9) | <0.0001 |
| Psychological disorders | 44.4±17.7 | 61.2±13.1 | 16.8 (10.2, 23.5) | <0.0001 |
Data are shown as mean ± SD. JOA, Japanese Orthopaedic Association; JOABPEQ, JOA Back Pain Evaluation Questionnaire; CI, confidence interval; SD, standard deviation.
Radiological measurements
The ICCs [2, 1] for intra-observer and inter-observer errors ranged between 0.73 and 0.949 and between 0.773 and 1, respectively. Consequently, all the measurements were considered reliable (Table 2). Preoperative and 2-year postoperative data for any radiological parameters were not significantly different (Table 3). No significant differences in the change in each parameter before and after surgery were found between groups D and ND (Table 4).
Table 2
| Radiological measurements | Intraobserver (95% CI) | Interobserver (95% CI) |
|---|---|---|
| Lateral translation | ||
| Preoperative | 0.762 (0.218, 0.936) | 0.861 (0.722, 0.933) |
| Postoperative | 0.883 (0.534, 0.971) | 0.773 (0.564, 0.888) |
| Sagittal translation | ||
| Preoperative | 0.849 (0.528, 0.96) | 1 |
| Postoperative | 0.949 (0.821, 0.987) | 0.986 (0.97, 0.993) |
| Disc wedging angle | ||
| Preoperative | 0.795 (0.349, 0.945) | 0.812 (0.633, 0.908) |
| Postoperative | 0.84 (0.482, 0.958) | 0.84 (0.653, 0.926) |
| Lordotic angle | ||
| Preoperative | 0.874 (0.461, 0.969) | 0.902 (0.801, 0.953) |
| Postoperative | 0.874 (0.461, 0.969) | 0.885 (0.745, 0.947) |
| Disc height | ||
| Preoperative | 0.73 (0.13, 0.93) | 0.921 (0.837, 0.962) |
| Postoperative | 0.874 (0.586, 0.967) | 0.776 (0.576, 0.889) |
ICC, intraclass correlation coefficients; CI, confidence interval.
Table 3
| Radiological measurements | Preoperative | Postoperative | Average of difference (95% CI) | P |
|---|---|---|---|---|
| Lateral translation (mm) | 1.0±1.9 | 1.2±1.9 | 0.2 (−0.4, 0.9) | 0.47 |
| Sagittal translation (mm) | 0.1±0.3 | 0.1±1.0 | −0.2 (−0.6, 0.2) | 0.31 |
| DST (mm) | 0.2±1.0 | 0.1±0.4 | −0.1 (−0.4, 0.1) | 0.20 |
| Disc wedging angle (°) | 1.8±2.6 | 1.7±3.3 | −0.1 (−1.2, 1.0) | 0.80 |
| Lordotic angle (°) | 8.8±4.5 | 7.9±4.9 | −0.9 (−2.4, 0.7) | 0.25 |
| DLA (°) | 6.2±3.2 | 4.9±3.2 | −1.3 (−2.6, 0.06) | 0.06 |
| Disc height (mm) | 7.9±2.4 | 7.6±2.4 | −0.3 (−0.8, 0.2) | 0.26 |
Data are shown as mean ± SD. CI, confidence interval; DST, difference in sagittal translation between flexion and extension; DLA, difference in lordotic angle between flexion and extension; SD, standard deviation.
Table 4
| Difference (postop.) – (preop.) | Group D | Group ND | P |
|---|---|---|---|
| Lateral translation (mm) | 0.1±1.7 | 0.4±0.5 | 0.81 |
| Sagittal translation (mm) | −0.4±0.4 | 0 | 0.12 |
| DST (mm) | −0.3±1.4 | 0 | 0.12 |
| Disc wedging angle (°) | −0.4±0.5 | 0.1±0.9 | 0.78 |
| Lordotic angle (°) | −1±1.4 | −0.8±0.7 | 0.70 |
| DLA (°) | −0.8±1.0 | −1.8±1.0 | 0.58 |
| Disc height (mm) | −0.2±0.4 | −0.3±0.2 | 0.66 |
Data are shown as mean ± SD. Group D, a disc group; Group ND, a non-disc group. preop, preoperative; postop, postoperative; DST, difference in sagittal translation between flexion and extension; DLA, difference in lordotic angle between flexion and extension; SD, standard deviation.
Discussion
Wide bony resection, including a certain part of the facet joint and pars interarticularis, is occasionally required for sufficient decompression of LFS, which carries the potential risk of postoperative segmental instability. However, attempts to preserve structural stability may result in insufficient decompression. The tradeoff between these two problems is sometimes difficult to resolve. Among the spinal levels, postoperative instability occurs much more frequently at L4–5 or upper spinal levels (3), whereas insufficient decompression is more often detected at L5–S1 (3,21). Effective surgical treatment for LFS at L5–S1 requires sufficient decompression; therefore, we primarily indicate “radical decompression” for patients with unilateral L5 radiculopathy due to LFS.
Decompression limited to the area of nerve compression may be effective in preserving the remaining bone tissue. Murata et al. investigated the localization of nerve root impingement in cases with symptomatic L5–S1 foraminal stenosis using 3D image fusion with MRI/computed tomography and found that the area for decompression should be extended to the intraforaminal region in approximately 75% of cases and to the extraforaminal region in approximately 80% of cases (22). Furthermore, an imaging study by Takahashi et al. revealed that more than half of the nerve root compressions in the extraforaminal region could be missed on conventional two-dimensional MRI, indicating that this was not an appropriate modality to specify the localization of nerve root compressions in patients with symptomatic foraminal stenosis at L5–S1 (23). Given that the localization of nerve compression is mediolaterally wide from the intraforaminal region to the extraforaminal region in many cases and that its exact localization is difficult to specify by the conventional modality, we believe that routine decompression from the intraforaminal region to the extraforaminal region is a simple method to obtain secure decompression and to minimize the risk of insufficient decompression. In our procedure, fenestration was first performed at L4–5 to identify the L5 nerve root and pedicle, which facilitates further process. Then the pars interarticularis, including the stable segment (24), was completely removed. Partial resection of the L5 transverse process and sacral ala was then performed in the extraforaminal region (11). The “radical decompression” procedure enables sufficient decompression from the intraforaminal region to the extraforaminal region and can be applied regardless of the localization of nerve root impingement.
In this study, radicular pain improved two weeks after surgery, and the JOA score significantly increased two years postoperatively. The JOA scores improved in all 28 patients, and none required revision surgery. In addition, JOABPEQ scores improved in all domains. Our “radical decompression” relieved not only leg symptoms but also low back pain caused by L5 radiculopathy.
Whether unilateral removal of the pars interarticularis leads to postoperative segmental instability remains under debate (25,26). Tender et al. stated that unilateral parsectomy is completely different from facetectomy because the former retains the inferior facet, which serves as a stabilizer (4). In addition, the iliolumbar ligaments bind the L5 transverse process to the ilium and stabilize the L5–S1 segment (10). Therefore, this segment should remain stable even after the unilateral removal of the pars interarticularis. In our study, we found no significant changes in any of the radiological parameters two years postoperatively. Furthermore, concerns arose regarding the impact of discectomy on segmental stability (27). Then, we classified the patients into two groups according to whether a discectomy was performed intraoperatively. No significant differences were observed between the groups. The “radical decompression” did not cause obvious segmental instability, and discectomy can be added if necessary.
Various types of surgeries for LFS have been reported (1,3,4,28-30), including microendoscopic decompression, unilateral biportal endoscopy, and PLIF. Microendoscopic decompression may be ideal because of its minimal invasiveness (6) and the usefulness of decompression of the extraforaminal region under a bright, magnified surgical field (6). Regrettably, this procedure is technically demanding, and the learning curve is rather steep (6). Unilateral biportal endoscopy is also minimally invasive; however, lower lumbar level (L4–5, L5–S1) is an independent risk factor for unsatisfactory outcomes (30). In addition, microendoscopy and biportal endoscopy are not available in every country. PLIF enables adequate decompression and stabilization simultaneously (5). However, it carries the risk of a higher rate of infection (7), nonunion at L5–S1 (8), and adjacent segment degeneration (31). Furthermore, a randomized controlled trial comparing the results of decompression alone to those of decompression with instrumented fusion for LFS found no significant additional benefits from fusion (32). Our “radical decompression” does not require specific equipment and devices, is not technically demanding, should have fewer complications, does not cause segmental stability, and offers secure decompression from the intraforaminal region to the extraforaminal region.
This study had several limitations. First, the amount of pars interarticularis removal was not standardized in the “radical decompression” procedure, and the bony resection at the pars interarticularis was widened as the L5–S1 disc was detected at the caudal area of the L5 nerve root. Therefore, the amount of remaining facet joint should be affected by the original size or swelling of the nerve root. Further analysis using postoperative computed tomography is needed to assess the relationship between the amount of remaining facet joints and postoperative segmental instability. Second, only one case of spondylolisthesis was included in this study, and the effect of preoperative sagittal translation on segmental stability could not be investigated. Therefore, it is unclear whether “radical decompression” causes segmental instability in patients with spondylolisthesis. Third, although the JOA score improved in all patients, two experienced a recovery rate of <25%. This study included only 28 patients, which may be too small a sample size to assess risk factors for poor surgical outcomes. Finally, two years may be too short a time frame to effectively assess the impact on spinal instability. Further studies with a larger number of patients and longer follow-up periods are warranted.
Conclusions
Our surgical technique resulted in good neurological recovery and was associated with a low risk of postoperative segmental instability, regardless of additional discectomy.
Acknowledgments
We would like to thank Editage for editing and reviewing this manuscript for the English language.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-23-62/rc
Data Sharing Statement: Available at https://jss.amegroups.com/article/view/10.21037/jss-23-62/dss
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-23-62/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-23-62/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Board of Tohoku Central Hospital (No. 107-1) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kunogi J, Hasue M. Diagnosis and operative treatment of intraforaminal and extraforaminal nerve root compression. Spine (Phila Pa 1976) 1991;16:1312-20. [Crossref] [PubMed]
- Ozeki N, Aota Y, Uesugi M, et al. Clinical results of intrapedicular partial pediculectomy for lumbar foraminal stenosis. J Spinal Disord Tech 2008;21:324-7. [Crossref] [PubMed]
- Yamada K, Matsuda H, Cho H, et al. Clinical and radiological outcomes of microscopic partial pediculectomy for degenerative lumbar foraminal stenosis. Spine (Phila Pa 1976) 2013;38:E723-31. [Crossref] [PubMed]
- Tender GC, Baratta RV, Voorhies RM. Unilateral removal of pars interarticularis. J Neurosurg Spine 2005;2:279-88. [Crossref] [PubMed]
- Watanabe K, Yamazaki A, Morita O, et al. Clinical outcomes of posterior lumbar interbody fusion for lumbar foraminal stenosis: preoperative diagnosis and surgical strategy. J Spinal Disord Tech 2011;24:137-41. [Crossref] [PubMed]
- Suzuki A, Nakamura H. Microendoscopic Lumbar Posterior Decompression Surgery for Lumbar Spinal Stenosis: Literature Review. Medicina (Kaunas) 2022;58:384. [Crossref] [PubMed]
- Ahn DK, Park HS, Choi DJ, et al. The difference of surgical site infection according to the methods of lumbar fusion surgery. J Spinal Disord Tech 2012;25:E230-4. [Crossref] [PubMed]
- Han SH, Hyun SJ, Jahng TA, et al. A Comparative Radiographic Analysis of Fusion Rate between L4-5 and L5-S1 in a Single Level Posterior Lumbar Interbody Fusion. Korean J Spine 2015;12:60-7. [Crossref] [PubMed]
- Jenis LG, An HS. Spine update. Lumbar foraminal stenosis. Spine (Phila Pa 1976) 2000;25:389-94. [Crossref] [PubMed]
- Aihara T, Takahashi K, Yamagata M, et al. Does the iliolumbar ligament prevent anterior displacement of the fifth lumbar vertebra with defects of the pars? J Bone Joint Surg Br 2000;82:846-50. [Crossref] [PubMed]
- Wiltse LL, Guyer RD, Spencer CW, et al. Alar transverse process impingement of the L5 spinal nerve: the far-out syndrome. Spine (Phila Pa 1976) 1984;9:31-41. [Crossref] [PubMed]
- Yamada H, Terada M, Iwasaki H, et al. Improved accuracy of diagnosis of lumbar intra and/or extra-foraminal stenosis by use of three-dimensional MR imaging: comparison with conventional MR imaging. J Orthop Sci 2015;20:287-94. [Crossref] [PubMed]
- Iwasaki M, Akiyama M, Koyanagi I, et al. Double Crush of L5 Spinal Nerve Root due to L4/5 Lateral Recess Stenosis and Bony Spur Formation of Lumbosacral Transitional Vertebra Pseudoarticulation: A Case Report and Review. NMC Case Rep J 2017;4:121-5. [Crossref] [PubMed]
- Kim HJ, Jeong JH, Cho HG, et al. Comparative observational study of surgical outcomes of lumbar foraminal stenosis using minimally invasive microsurgical extraforaminal decompression alone versus posterior lumbar interbody fusion: a prospective cohort study. Eur Spine J 2015;24:388-95. [Crossref] [PubMed]
- Baba H, Uchida K, Maezawa Y, et al. Microsurgical nerve root canal widening without fusion for lumbosacral intervertebral foraminal stenosis: technical notes and early results. Spinal Cord 1996;34:644-50. [Crossref] [PubMed]
- Hashimoto K, Tanaka Y, Tsubakino T, et al. Imaging diagnosis of lumbar foraminal stenosis in the fifth lumbar nerve root: reliability and reproducibility of T1-weighted three-dimensional lumbar MRI. J Spine Surg 2021;7:502-9. [Crossref] [PubMed]
- Aizawa T, Ozawa H, Kusakabe T, et al. Reoperation rates after fenestration for lumbar spinal canal stenosis: a 20-year period survival function method analysis. Eur Spine J 2015;24:381-7. [Crossref] [PubMed]
- Wiltse LL, Bateman JG, Hutchinson RH, et al. The paraspinal sacrospinalis-splitting approach to the lumbar spine. J Bone Joint Surg Am 1968;50:919-26.
- Hirabayashi K, Watanabe K, Wakano K, et al. Expansive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine (Phila Pa 1976) 1983;8:693-9. [Crossref] [PubMed]
- Fukui M, Chiba K, Kawakami M, et al. JOA Back Pain Evaluation Questionnaire (JOABPEQ)/JOA Cervical Myelopathy Evaluation Questionnaire (JOACMEQ). The report on the development of revised versions. April 16, 2007. The Subcommittee of the Clinical Outcome Committee of the Japanese Orthopaedic Association on Low Back Pain and Cervical Myelopathy Evaluation. J Orthop Sci 2009;14:348-65. [Crossref] [PubMed]
- Bae JS, Kang KH, Park JH, et al. Postoperative Clinical Outcome and Risk Factors for Poor Outcome of Foraminal and Extraforaminal Lumbar Disc Herniation. J Korean Neurosurg Soc 2016;59:143-8. [Crossref] [PubMed]
- Murata S, Minamide A, Iwasaki H, et al. Microendoscopic decompression for lumbosacral foraminal stenosis: a novel surgical strategy based on anatomical considerations using 3D image fusion with MRI/CT. J Neurosurg Spine 2020; Epub ahead of print. [Crossref]
- Takahashi K, Latt MM, Tsubakino T, et al. Reliability of conventional two-dimensional magnetic resonance imaging for diagnosing extraforaminal stenosis in lumbosacral transition. Spine Surgery and Related Research 2023; [Crossref]
- Laurencin CT, Lipson SJ, Senatus P, et al. The stenosis ratio: a new tool for the diagnosis of degenerative spinal stenosis. Int J Surg Investig 1999;1:127-31.
- Enyo Y, Yamada H, Kim JH, et al. Microendoscopic lateral decompression for lumbar foraminal stenosis: a biomechanical study. J Spinal Disord Tech 2014;27:257-62. [Crossref] [PubMed]
- Tender GC, Kutz S, Baratta R, et al. Unilateral progressive alterations in the lumbar spine: a biomechanical study. J Neurosurg Spine 2005;2:298-302. [Crossref] [PubMed]
- Schaller B. Failed back surgery syndrome: the role of symptomatic segmental single-level instability after lumbar microdiscectomy. Eur Spine J 2004;13:193-8.
- Chang HS, Zidan I, Fujisawa N, et al. Microsurgical posterolateral transmuscular approach for lumbar foraminal stenosis. J Spinal Disord Tech 2011;24:302-7. [Crossref] [PubMed]
- Kang K, Rodriguez-Olaverri JC, Schwab F, et al. Partial facetectomy for lumbar foraminal stenosis. Adv Orthop 2014;2014:534658. [Crossref] [PubMed]
- You KH, Kang MS, Lee WM, et al. Biportal endoscopic paraspinal decompressive foraminotomy for lumbar foraminal stenosis: clinical outcomes and factors influencing unsatisfactory outcomes. Acta Neurochir (Wien) 2023;165:2153-63. [Crossref] [PubMed]
- Hashimoto K, Aizawa T, Kanno H, et al. Adjacent segment degeneration after fusion spinal surgery-a systematic review. Int Orthop 2019;43:987-93. [Crossref] [PubMed]
- Hallett A, Huntley JS, Gibson JN. Foraminal stenosis and single-level degenerative disc disease: a randomized controlled trial comparing decompression with decompression and instrumented fusion. Spine (Phila Pa 1976) 2007;32:1375-80. [Crossref] [PubMed]


