Development and validation of a point-of-care clinical risk score to predict surgical site complication-associated readmissions following open spine surgery
Highlight box
Key findings
• A 19-factor risk assessment score has been developed and validated to predict surgical site complication-associated readmissions (SSC-ARs) in adult patients (n=157,664; 3,182 SSC-ARs) who underwent open spine surgery, demonstrating strong discriminatory capability (C-statistic =74.12%).
What is known and what is new?
• Existing universal calculators for SSC risk often overlook important factors specific to spine procedures and have suboptimal predictive ability for unplanned readmissions.
• This study employed a comprehensive definition of wound-related SSC, identified risk factors specifically relevant to open spine surgery, and developed a model particularly for SSC-ARs, differentiating it from previous prediction models for postoperative complications.
What is the implication, and what should change now?
• Accurately predicting wound-related surgical complications based on preoperative factors is crucial for reducing unplanned readmissions and enhancing value-based care in open spine surgery. An economic model is required to validate this conclusion.
IntroductionOther Section
Healthcare utilization and expenditures for spine surgery continue to rise in the United States (US). The rate of complex spine surgery demonstrated a nearly 15-fold increase in the US Medicare population from 2002 to 2007 (1), and the national bill for spinal fusion increased from $10 billion to $46.8 billion with an estimated 3.6 million spine fusions occurring between 2001 and 2010 (2). As of 2021, this number had grown to 1.6 million spinal fusions annually in the US (3). The escalating rate of spine surgeries and associated costs emphasizes the need to address reducible expenses, including preventable wound complications and readmissions, to enhance healthcare efficiency and surgical outcomes (4,5).
Preventable and unplanned readmissions contribute to substantial financial burdens, costing Medicare up to approximately $12 billion per year (6,7). Among the leading causes of such readmissions following open spine surgery are wound-related and surgical site complications (SSCs) (8,9). These SSCs encompass complications like wound dehiscence, surgical site infections (SSIs), hematoma, seroma, and skin necrosis resulting from impaired wound healing (5,10,11). SSIs, in particular, are the most prevalent wound-related complications following spine surgery and have been associated with increased mortality rates, pseudoarthrosis, neurologic injury, readmission rates, and extended hospital stays (12-16). A meta-analysis of 161 studies from North America, Europe, and Asia, showed that spine surgery patients approximately double the hospital length of stay and health care costs when they developed an SSI (17). Dehiscence, the second most common SSC following spine surgery, also adds to the economic burden by necessitating additional wound-related operations (18).
The reported incidence of SSCs following spinal surgery, including wound dehiscence and SSI, ranges from 2% to 20% (5,14,19,20). A systematic review and meta-analysis of data from 2000 to 2015 indicated a 5.5% [95% confidence interval (CI): 4.2–7.4%] 30-day readmission rate for spine surgeries in the US (21). However, the literature regarding the incidence and risk factors associated with SSC-induced readmissions after open spine surgery is limited. Understanding these factors could offer insights into mitigating the risk of readmissions caused by severe SSCs. Hospitals face challenges in determining cost-effective strategies to mitigate complication-associated readmissions, particularly when the overall readmission rate for spine surgery is not excessively high. Implementing expensive interventions for a large patient population may not be economically justified.
Preoperative assessment tools, such as the American College of Surgeons Surgical Risk Calculator (ACS-SRC) (22) and the Surgical Risk Preoperative Assessment System (SURPAS) (23-25), are available for predicting postoperative complications. While these tools demonstrate effectiveness in predicting mortality, urinary tract infection, cardiac, venous thromboembolism, and renal complications, their ability to predict unplanned readmissions is suboptimal (26). Therefore, there is a need for a user-friendly assessment tool that accurately stratifies the risk of readmission induced by surgical complications. This tool would enable preoperative identification of the subset of patients who are more susceptible and may benefit from risk optimization or advanced incisional management strategies.
The objective of this study was to develop and validate a predictive scoring system to quantify the readmission risk associated with SSCs following open spine surgeries. The ultimate goal of this point-of-care clinical tool was to establish a risk level continuum for experiencing an unplanned SSC-associated readmission (SSC-AR). We present this article in accordance with the TRIPOD reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-23-89/rc).
MethodsOther Section
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Data source
This study utilized data obtained from the Premier Healthcare Database (PHD) encompassing adult patients, from 599 US hospitals, who underwent open spine surgery between January 1, 2019, and September 30, 2020. The PHD is an extensive all-payer database that captures information on inpatient discharges from a wide range of geographically diverse non-profit, non-governmental, community, and teaching hospitals and health systems across the US (27). This study utilized deidentified data in accordance with 45 CFR 164.506(d)(2)(ii)(B), employing the “Expert Determination” method (27), and was exempt from institutional review board review and the requirement for informed consent.
Study population
A total of 158,664 complete records of open spine surgeries were identified using a series of 463 pre-identified International Classification of Diseases (ICD)-10 procedure codes. Among these records, 3,209 cases (2.0%) were classified as having at least one SSC-AR within 90 days of discharge, based on the presence of relevant ICD-10 diagnosis codes at admission. Interested readers can refer to a Table S1 for a list of medical codes used to identify SSCs. Surgeries involving planned durotomies, patients under the age of eighteen, or utilization of negative pressure therapy over the closed incision were excluded from the analysis. The 158,664 complete records contained no missing data for the selected variables.
An independent validation cohort was established, consisting of a randomly selected subset of 1,000 cases (27 with an SSC-AR) out of the total 158,664 records. The development cohort comprised the remaining 157,664 records, with 3,182 cases of SSC-ARs, and was utilized for the main SSC-AR prediction. The records in the development cohort encompassed various payor types, including Medicare (49.0%), managed care (23.7%), Medicaid (9.1%), commercial (8.8%), and self-pay (1.2%). The validation dataset exhibited a slightly higher SSC-AR rate compared to the development dataset (2.7% vs. 2.0%). There were no differences in the setting, eligibility, or predictor variables between the development and validation datasets.
Outcome variable
The outcome variable in this study was measured as a binary variable indicating whether a patient experienced an SSC-AR following spine surgery. SSC was defined as wound-related and encompassed SSIs, dehiscences, seromas, skin complications, and other non-healing wounds based on reported literature (2,11,22,23,28,29). For this study, records with readmissions within 90 days after discharge from the index spine surgery were examined. A readmission with primary diagnosis of one or more SSCs was classified as having occurrence of an SSC. The occurrence and type of SSC were determined using ICD-10 codes and diagnosis-related group (DRG) codes.
Risk factors
Patient-, facility-, and procedure-related risk factors known to be predictors of higher SSC rates after spine surgeries (2,5,11,28,30-32) were measured as independent variables. The spine surgical invasiveness index (33,34), a composite metric evaluating the invasiveness of the surgical approach (anterior/posterior), surgical modality (decompression/fusion/instrumentation), and vertebral levels (thoracic/lumbar/sacral) concurrently, was computed as a proximate estimate based on procedure codes listed for each patient within the scope of this study. Patient factors included age, gender, comorbidities, and health behaviors (e.g., alcohol disorder, cocaine disorder, and nicotine dependence). Comorbidities and health behaviors were determined via ICD-10 diagnosis codes. Number of hospital beds, hospital cost type (procedural vs. ratio of costs-to-charges), and facility geographical location were used to measure facility risk.
Procedure-specific factors considered influential to SSC in this study were the following: primary/revision surgery, blood transfusion, surgery complexity (number of ICD-10 procedures and operative time), surgery case urgency, spine region location of operation, and the surgical invasiveness index as a proximate estimate based on procedure codes listed for each patient. Each surgery’s spinal region risk score was calculated as the sum of the points assigned to three different spinal regions (cervical =1, lumbosacral =2, and thoracic =3). This scoring methodology was derived from existing literature, which provides compelling evidence that the anatomical location of spine surgery associated with the risk of SSI, following this hierarchical order: thoracic procedures > lumbosacral/lumbar procedures > cervical procedures (34,35). Most records did not include weight, body mass index, or lab values, and therefore, this data was not extracted for analysis.
Statistical analysis
A mixed effects logistic regression model was developed as a foundation for a scoring algorithm of SSC-AR risk using a development cohort (n=157,664; 3,182 SSCs). Initially, a full multilevel logistic regression model was constructed to determine the association between SSC-AR and 37 predictors identified from the PHD dataset (Table 1). To account for facility-level clustering, a random effect was added to the intercept. Subsequently, a reduced multilevel logistic regression model was developed using the 19 strongest predictors from the full model (Table 2). Discriminatory capability and goodness of fit were compared between the full and reduced models.
Table 1
| Risk factors | SSC-AR risk full model | Development cohort | Validation cohort | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | P value | OR (95% CI) | Overall (n=157,664) |
No SSC-AR (n=154,482) | SSC-AR (n=3,182) | Overall (n=1,000) |
|||
| Patient demographics | ||||||||||
| Age (years) | 0.01 | 0.00 | 0.0007 | 1.00 (0.99, 1.00) | 60.8±13.5 | 60.8±13.5 | 61.8±12.9 | 61.7±13.6 | ||
| Gender: F | 0.33 | 0.07 | <0.0001 | 1.22 (1.13, 1.31) | 79,889 (50.7) | 78,148 (50.6) | 1,741 (54.7) | 511 (51.1) | ||
| Gender: M | Ref. | Ref. | Ref. | – | 77,775 (49.3) | 76,334 (49.4) | 1,441 (45.3) | 489 (48.9) | ||
| Patient comorbidities | ||||||||||
| Asthma | 0.28 | 0.08 | 0.0004 | 0.75 (0.64, 0.88) | 14,377 (9.1) | 14,019 (9.1) | 358 (11.3) | 86 (8.6) | ||
| Blood disorder† | 0.18 | 0.04 | <0.0001 | 1.19 (1.10, 1.30) | 39,160 (24.8) | 37,914 (24.5) | 1,246 (39.2) | 241 (24.1) | ||
| Myocardial infarction | 0.11 | 0.07 | 0.1038 | 1.11 (0.98, 1.27) | 9,575 (6.1) | 9,247 (6.0) | 328 (10.3) | 62 (6.2) | ||
| Paraplegia and hemiplegia | 0.44 | 0.06 | <0.0001 | 1.56 (1.38, 1.75) | 9,678 (6.1) | 9,247 (6.0) | 431 (13.5) | 54 (5.4) | ||
| Renal disease | 0.36 | 0.06 | <0.0001 | 1.44 (1.27, 1.63) | 15,078 (9.6) | 14,478 (9.4) | 600 (18.9) | 88 (8.8) | ||
| Cancer | 0.22 | 0.08 | 0.0044 | 1.24 (1.07, 1.44) | 7,216 (4.6) | 6,925 (4.5) | 291 (9.1) | 58 (5.8) | ||
| AIDS/HIV | 0.52 | 0.27 | 0.0568 | 1.68 (0.99, 2.85) | 312 (0.2) | 296 (0.2) | 16 (0.5) | 0 (0.0) | ||
| Congestive heart failure | 0.35 | 0.06 | <0.0001 | 1.42 (1.26, 1.60) | 11,056 (7.0) | 10,550 (6.8) | 506 (15.9) | 67 (6.7) | ||
| Peripheral vascular disease | 0.28 | 0.06 | <0.0001 | 1.32 (1.17, 1.48) | 10,266 (6.5) | 9,875 (6.4) | 391 (12.3) | 75 (7.5) | ||
| Cerebrovascular disease | 0.00 | 0.07 | 0.9498 | 1.01 (0.87, 1.16) | 7,116 (4.5) | 6,869 (4.4) | 247 (7.8) | 39 (3.9) | ||
| Dementia | 0.47 | 0.10 | <0.0001 | 1.60 (1.32, 1.93) | 2,995 (1.9) | 2,856 (1.8) | 139 (4.4) | 23 (2.3) | ||
| Chronic pulmonary disease | 0.56 | 0.06 | <0.0001 | 1.74 (1.54, 1.98) | 34,821 (22.1) | 33,755 (21.9) | 1,066 (33.5) | 225 (22.5) | ||
| Rheumatic disease | 0.51 | 0.06 | <0.0001 | 1.66 (1.47, 1.88) | 7,959 (5.0) | 7,648 (5.0) | 311 (9.8) | 51 (5.1) | ||
| Peptic ulcer disease | 0.11 | 0.14 | 0.4336 | 1.12 (0.85, 1.46) | 1,541 (1.0) | 1,483 (1.0) | 58 (1.8) | 6 (0.6) | ||
| COPD | 0.36 | 0.08 | <0.0001 | 0.70 (0.60, 0.81) | 13,798 (8.8) | 13,390 (8.7) | 408 (12.8) | 93 (9.3) | ||
| Diabetes | 0.32 | 0.04 | <0.0001 | 1.37 (1.26, 1.49) | 41,456 (26.3) | 40,169 (26.0) | 1,287 (40.4) | 248 (24.8) | ||
| Liver disease | 0.33 | 0.07 | <0.0001 | 1.39 (1.21, 1.59) | 6,574 (4.2) | 6,302 (4.1) | 272 (8.5) | 51 (5.1) | ||
| Obesity | 0.34 | 0.04 | <0.0001 | 1.40 (1.29, 1.51) | 38,439 (24.4) | 37,344 (24.2) | 1,095 (34.4) | 233 (23.3) | ||
| Alcohol disorder | 0.15 | 0.11 | 0.1642 | 1.16 (0.94, 1.43) | 3,857 (2.4) | 3,750 (2.4) | 107 (3.4) | 26 (2.6) | ||
| Hypertension | 0.26 | 0.04 | <0.0001 | 1.29 (1.19, 1.40) | 80,961 (51.4) | 79,229 (51.3) | 1,732 (54.4) | 515 (51.5) | ||
| Cocaine disorder | 0.15 | 0.27 | 0.5834 | 0.86 (0.50, 1.47) | 581 (0.4) | 566 (0.4) | 15 (0.5) | 3 (0.3) | ||
| Nicotine dependence | 0.11 | 0.05 | 0.0328 | 1.11 (1.01, 1.23) | 24,887 (15.8) | 24,304 (15.7) | 583 (18.3) | 151 (15.1) | ||
| Severe comorbidity | 0.23 | 0.07 | 0.0015 | 1.26 (1.09, 1.45) | 15,555 (9.9) | 14,724 (9.5) | 831 (26.1) | 95 (9.5) | ||
| Surgery-related risk | ||||||||||
| Blood transfusion | 0.14 | 0.07 | 0.0458 | 1.15 (1.00, 1.32) | 7,454 (4.7) | 7,119 (4.6) | 335 (10.5) | 44 (4.4) | ||
| Invasiveness index | 0.03 | 0.00 | <0.0001 | 1.03 (1.02, 1.04) | 4.4±3.9 | 4.4±3.9 | 5.7±5.2 | 4.7±4.2 | ||
| Surgery type: revision | 0.27 | 0.05 | <0.0001 | 1.32 (1.20, 1.44) | 31,843 (20.2) | 30,994 (20.1) | 849 (26.7) | 181 (18.1) | ||
| Surgery type: primary | Ref. | Ref. | Ref. | – | 125,821 (79.8) | 123,488 (79.9) | 2,333 (73.3) | 819 (81.9) | ||
| Spinal region risk score | 0.01 | 0.01 | 0.3354 | 1.01 (0.99, 1.03) | 3.9±2.2 | 3.9±2.2 | 4.2±2.4 | 3.8±2.2 | ||
| Number of ICD procedures ≥10 | 0.03 | 0.08 | 0.7160 | 0.97 (0.83, 1.14) | 5,409 (3.4) | 5,177 (3.4) | 232 (7.3) | 39 (3.9) | ||
| Emergency/urgent surgery | 0.22 | 0.05 | <0.0001 | 1.24 (1.13, 1.36) | 29,926 (19.0) | 28,993 (18.8) | 933 (29.3) | 195 (19.5) | ||
| Operative hours ≥5 | 0.52 | 0.04 | <0.0001 | 1.68 (1.55, 1.83) | 42,803 (27.1) | 41,455 (26.8) | 1,348 (42.4) | 283 (28.3) | ||
| Facility-related risk | ||||||||||
| Number of beds: 100–199 | 0.29 | 0.17 | 0.0939 | 1.33 (0.95, 1.86) | 17,912 (11.4) | 17,623 (11.4) | 289 (9.1) | 113 (11.3) | ||
| Number of beds: 200–299 | 0.35 | 0.17 | 0.0391 | 1.41 (1.02, 1.96) | 22,970 (14.6) | 22,543 (14.6) | 427 (13.4) | 151 (15.1) | ||
| Number of beds: 300–399 | 0.40 | 0.17 | 0.0166 | 1.49 (1.08, 2.08) | 26,337 (16.7) | 25,807 (16.7) | 530 (16.7) | 161 (16.1) | ||
| Number of beds: 400–499 | 0.45 | 0.17 | 0.0095 | 1.57 (1.12, 2.21) | 20,120 (12.8) | 19,714 (12.8) | 406 (12.8) | 128 (12.8) | ||
| Number of beds: 500+ | 0.42 | 0.17 | 0.0131 | 1.52 (1.09, 2.11) | 64,287 (40.8) | 62,825 (40.7) | 1,462 (45.9) | 406 (40.6) | ||
| Number of beds: 000–099 | Ref. | Ref. | Ref. | – | 6,038 (3.8) | 5,970 (3.9) | 68 (2.1) | 41 (4.1) | ||
| Cost type: procedural | 0.03 | 0.07 | 0.6958 | 0.97 (0.85, 1.11) | 110,508 (70.1) | 108,235 (70.1) | 2,273 (71.4) | 681 (68.1) | ||
| Cost type: RCC | Ref. | Ref. | Ref. | – | 47,156 (29.9) | 46,247 (29.9) | 909 (28.6) | 319 (31.9) | ||
| Provider region: Midwest | 0.10 | 0.10 | 0.3275 | 0.91 (0.75, 1.10) | 37,012 (23.5) | 36,325 (23.5) | 687 (21.6) | 233 (23.3) | ||
| Provider region: South | 0.14 | 0.09 | 0.1302 | 1.15 (0.96, 1.37) | 74,794 (47.4) | 73,230 (47.4) | 1,564 (49.2) | 486 (48.6) | ||
| Provider region: West | 0.24 | 0.11 | 0.0335 | 1.27 (1.02, 1.58) | 18,261 (11.6) | 17,853 (11.6) | 408 (12.8) | 124 (12.4) | ||
| Provider region: Northeast | Ref. | Ref. | Ref. | – | 27,597 (17.5) | 27,074 (17.5) | 523 (16.4) | 157 (15.7) | ||
| Provider location: rural | 0.11 | 0.10 | 0.2819 | 1.11 (0.92, 1.36) | 13,186 (8.4) | 12,908 (8.4) | 278 (8.7) | 93 (9.3) | ||
| Provider location: urban | Ref. | Ref. | Ref. | – | 144,478 (91.6) | 141,574 (91.6) | 2,904 (91.3) | 907 (90.7) | ||
| Teaching hospital | 0.12 | 0.07 | 0.0788 | 1.13 (0.99, 1.30) | 86,771 (55.0) | 84,860 (54.9) | 1,911 (60.1) | 556 (55.6) | ||
Data are presented as n (%) or mean ± SD. †, blood disorder: diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism. SSC-AR, surgical site complication-associated readmission; SE, standard error; OR, odds ratio; CI, confidence interval; F, female; M, male; Ref., reference; AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus; COPD, chronic obstructive pulmonary disease; ICD, International Classification of Diseases; RCC, ratio of costs-to-charges (to pay hospitals for services exempt from DRG payment); DRG, diagnosis-related group; SD, standard deviation.
Table 2
| Reduced model | β | SE | P value | OR (95% CI) | Score [0–40] |
|---|---|---|---|---|---|
| Patient-related risk | |||||
| Female gender | 0.20 | 0.04 | <0.0001 | 1.22 (1.14, 1.32) | 1 |
| Blood disorder† | 0.24 | 0.04 | <0.0001 | 1.28 (1.18, 1.38) | 2 |
| Congestive heart failure | 0.33 | 0.06 | <0.0001 | 1.40 (1.24, 1.57) | 2 |
| Dementia | 0.40 | 0.09 | <0.0001 | 1.49 (1.24, 1.80) | 3 |
| Chronic pulmonary disease | 0.31 | 0.04 | <0.0001 | 1.36 (1.26, 1.47) | 2 |
| Rheumatic disease | 0.50 | 0.06 | <0.0001 | 1.65 (1.46, 1.87) | 3 |
| Hypertension | 0.24 | 0.04 | <0.0001 | 1.27 (1.17, 1.38) | 2 |
| Obesity | 0.34 | 0.04 | <0.0001 | 1.40 (1.29, 1.51) | 2 |
| Severe comorbidity (CCI ≥5) | 0.29 | 0.07 | <0.0001 | 1.34 (1.17, 1.54) | 2 |
| Nicotine dependence | 0.15 | 0.05 | 0.0016 | 1.17 (1.06, 1.28) | 1 |
| Liver disease | 0.37 | 0.07 | <0.0001 | 1.44 (1.26, 1.65) | 2 |
| Paraplegia and hemiplegia | 0.46 | 0.06 | <0.0001 | 1.59 (1.41, 1.79) | 3 |
| Peripheral vascular disease | 0.27 | 0.06 | <0.0001 | 1.31 (1.16, 1.47) | 2 |
| Renal disease | 0.30 | 0.06 | <0.0001 | 1.35 (1.20, 1.53) | 2 |
| Cancer | 0.21 | 0.07 | 0.0051 | 1.23 (1.06, 1.42) | 1 |
| Diabetes | 0.29 | 0.04 | <0.0001 | 1.34 (1.23, 1.45) | 2 |
| Surgery-related risk | |||||
| Revision surgery‡ | 0.26 | 0.04 | <0.0001 | 1.30 (1.20, 1.42) | 2 |
| Operative hours ≥5 | 0.65 | 0.04 | <0.0001 | 1.92 (1.77, 2.07) | 4 |
| Emergency/urgent surgery | 0.23 | 0.05 | <0.0001 | 1.26 (1.15, 1.38) | 2 |
| Risk score model in the study population (n=157,664, 3,182 SSC-ARs) | |||||
| SSC-AR risk score | 0.15 | 0.00 | <0.0001 | 1.16 (1.15, 1.17) | – |
| Risk score model in the random sample (n=1,000, 27 SSC-ARs) | |||||
| SSC risk score | 0.16 | 0.04 | <0.0001 | 1.18 (1.09, 1.27) | – |
†, blood disorder: diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism; ‡, primary surgery as the reference. SSC-AR, surgical site complication-associated readmission; SE, standard error; OR, odds ratio; CI, confidence interval; CCI, Charlson Comorbidity Index.
A risk-assessment point scoring system was then developed from the 19 predictors in the reduced model to weight each factor based on its effect size in association with SSC-AR. Points were assigned based on the relative strength of each factor: the smallest β coefficient (0.1526) was assigned one point, and each other risk factor was assigned a score (rounded to integer) by dividing its β by 0.1526 (36). The resulting risk score was the sum of points assigned to the 19 individual predictors (Table 2).
The discriminatory capability of the two models was assessed by receiver operating characteristic (ROC) curve analysis. Goodness of fit was assessed via a chi-square statistic [chi-square/degrees of freedom (DF)]. The deviance or Pearson’s chi-square divided by its DF served as an estimate of the dispersion parameter Փ, assessing goodness of fit of a given generalized linear model using GENMOD procedure (SAS 9.4; SAS Institute, Cary, NC, USA) (37). ROC of the full model served as a benchmark for comparison to determine whether the reduced model was an equally strong prediction of SSC-AR. A logistic regression model with the risk score as the sole predictor was fitted in both the development cohort and a holdout sample of 1,000 random observations (27 with SSC-AR) independent from the development cohort for validation of the risk score.
ResultsOther Section
Descriptive statistics
Table 1 presents the results of the SSC-AR full model, along with descriptive statistics, n (%) or mean ± standard deviation (SD), for each risk factor in both the development and validation cohorts. Additionally, the descriptive statistics for the development cohort are further categorized into SSC-AR and no SSC-AR groups. The development cohort comprised of 3,182 (2.0%) cases with SSC-ARs (“SSC-AR” group) and 154,482 (98.0%) cases without (“no SSC-AR” group) (Table 1). The types and frequencies of SSCs requiring readmission were SSI (67.5%), dehiscence (44.1%), postoperative seroma (13.7%), skin complication (10.8%), and other non-healing wound (4.7%). The average age of open spine patients at the time of initial surgery was 60.8 (range, 18–89) years old, and 50.7% of the study cohort were female. A relatively high proportion of the cohort were obese (24.4%) and/or hypertensive (51.4%). Blood disorders affected 24.8% of the population, 15.8% were current smokers, and 22.1% had chronic pulmonary disease. A total of 29,926 (19.0%) cases were classified as emergent/urgent. Twenty percent of patients underwent revision surgeries, and 79.8% primary surgeries, which were composed of 78.7% primary fusion, 8.8% revision fusion, 0.8% primary non-fusion, and 11.7% revision non-fusion surgeries. The primary fusion surgeries consisted of posterolateral (28.0%), anterior (26.9%), posterior interbody and lateral (23.1%), posterior interbody (13.7%), and anterior and posterior combined (8.3%) approaches. The average operative time was 4.2 hours (SD =2.3), with 27.1% of the surgeries lasting 5 hours or longer. Nearly 10% of the patients had severe comorbidities with a Charlson comorbidity score ≥5. The SSC-AR group exhibited a higher percentage of risk factors compared to the no SSC-AR group, particularly in relation to severe comorbidities (26.1% vs. 9.5%), paraplegia and hemiplegia (13.5% vs. 6.0%), renal disease (18.9% vs. 9.4%), and diabetes (40.4% vs. 26.0%). The validation cohort demonstrated a distribution of risk factors comparable to that of the development cohort (Table 1).
Full model
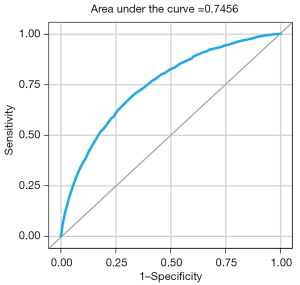
The full prediction model, incorporating all 37 predictors, demonstrated good discriminatory capability [C-statistic =74.56%; 95% confidence interval (CI): 73.70–75.42%] (Figure 1), as well as a satisfactory model fit (chi-square/DF =0.92). The effect size, significance, and odds ratio (OR) of each predictor are listed in Table 1. All 37 predictors are preoperative except “blood transfusion” which was not selected for the reduced model. Among the most influential risk factors for SSC-AR were chronic pulmonary disease (OR, 1.7; 95% CI: 1.5 to 2.0), operative hours ≥5 (OR, 1.7; 95% CI: 1.6 to 1.8), dementia (OR, 1.6; 95% CI: 1.3 to 1.9), and paraplegia/hemiplegia (OR, 1.6; 95% CI: 1.4 to 1.8).
Reduced model and SSC-AR risk score
A reduced model was developed by selecting the 19 strongest predictors (Table 2). Patients with rheumatic disease (OR, 1.7; 95% CI: 1.5 to 1.9) and paraplegia/hemiplegia (OR, 1.6; 95% CI: 1.4 to 1.8) at baseline, as well as those who underwent a surgery of 5 hours or longer (OR, 1.9; 95% CI: 1.8 to 2.1) were more likely to experience SSC-ARs. Dementia (OR, 1.5; 95% CI: 1.2 to 1.8) and liver disease (OR, 1.4; 95% CI: 1.3 to 1.7) also considerably increased the probability of SSC-AR.
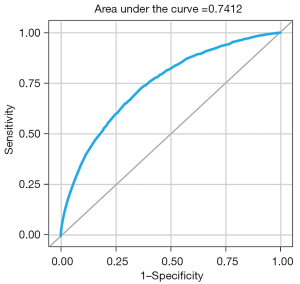
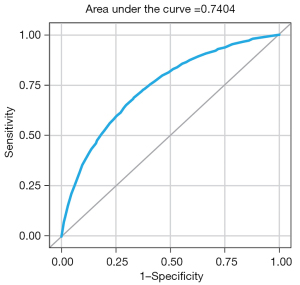
The reduced model exhibited fair discriminative power (C-statistic =74.12%; 95% CI: 73.26–74.98%) (Figure 2) and better model fit (Pearson chi-square/DF =0.93) compared to the full model. The reduced model’s better fit over the full model was further supported by the calculated consistent Akaike information criterion (CAIC) (38), an estimator of prediction error and of statistical model quality. The CAIC was slightly lower in the reduced model vs. the full model (29,329 vs. 29,361, respectively).
Risk factor variables and their weights derived from the reduced model were the following: female gender (1 point), blood disorder [2], congestive heart failure [2], dementia [3], chronic pulmonary disease [2], rheumatic disease [3], hypertension [2], obesity [2], severe comorbidity [2], nicotine dependence [1], liver disease [2], paraplegia and hemiplegia [3], peripheral vascular disease [2], renal disease [2], cancer [1], diabetes [2], revision surgery [2], operative hours ≥5 [4], and emergency/urgent surgery [2].
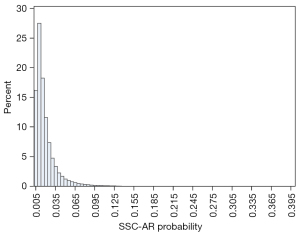
The total SSC-AR risk score, as the sum of the weights assigned to the planned procedure and all baseline comorbidities, had a possible score ranging from 0 to 40. Each specific risk score corresponds to an estimated probability of SSC-AR. As an example, an obese (2 points), hypertensive (2 points) patient with a blood disorder (2 points) and renal disease (2 points) who underwent an urgent (2 points) 6-hour (4 points) revision surgery (2 points) would have a total risk score of 16 points (Table 2) and an estimated probability of SSC-AR of 8.5% (Table 3). Most surgery cases had a relatively low estimated risk of SSC-AR; 99% had a risk score <20; only one case was at a markedly elevated risk with a score >30 (Table 3). The distribution of risk scores for this primary study cohort is presented in Figure 3. The probability of risk mirrored this distribution: 97% of the population had an SSC-AR risk lower than 7.0%, while 0.1% had a high risk (≥18%) (Table 4, Figure 4).
Table 3
| Risk score as a cutoff | Probability of SSC-AR (%) | Surgeries (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| 0 | 2.0 | 100.0 | 100.0 | 0.0 | 2.0 | – |
| 2 | 2.1 | 90.9 | 97.5 | 9.2 | 2.2 | 99.4 |
| 4 | 2.4 | 74.5 | 90.5 | 25.9 | 2.5 | 99.3 |
| 6 | 2.8 | 54.9 | 80.0 | 45.7 | 2.9 | 99.1 |
| 8 | 3.5 | 37.1 | 66.4 | 63.5 | 3.6 | 98.9 |
| 10 | 4.3 | 23.1 | 51.9 | 77.5 | 4.5 | 98.7 |
| 12 | 5.4 | 13.6 | 37.6 | 86.9 | 5.6 | 98.5 |
| 14 | 6.9 | 7.6 | 25.1 | 92.8 | 6.7 | 98.4 |
| 16 | 8.5 | 4.1 | 16.0 | 96.1 | 7.8 | 98.2 |
| 18 | 10.5 | 2.0 | 8.9 | 98.1 | 8.8 | 98.1 |
| 20 | 13.1 | 0.9 | 4.0 | 99.2 | 8.9 | 98.0 |
| 22 | 16.3 | 0.4 | 1.8 | 99.7 | 10.5 | 98.0 |
| 24 | 20.4 | 0.1 | 0.6 | 99.9 | 11.1 | 98.0 |
SSC-AR, surgical site complication-associated readmission; PPV, positive predictive value; NPV, negative predictive value.
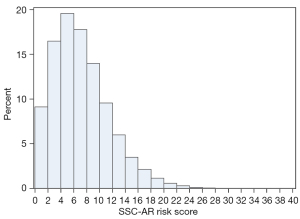
Table 4
| Predicted SSC-AR risk (%) | Number of surgeries (%) |
Number of SSC-ARs predicted | Actual number of SSC-ARs (%) |
% Total SSC-ARs (n=3,182) |
|---|---|---|---|---|
| <3 | 132,133 (83.8) | 1,780 | 1,704 (1.3) | 53.6 |
| 3–6.9 | 21,152 (13.4) | 905 | 1,027 (4.9) | 32.3 |
| 7.0–8.9 | 2,097 (1.3) | 165 | 191 (9.1) | 6.0 |
| 9.0–12.9 | 1,538 (1.0) | 163 | 157 (10.2) | 4.9 |
| 13.0–17.9 | 536 (0.3) | 80 | 72 (13.4) | 2.3 |
| 18.0–27.9 | 184 (0.1) | 39 | 23 (12.5) | 0.7 |
| 28.0–37.9 | 23 (0.0) | 7 | 8 (34.8) | 0.3 |
| ≥38.0 | 1 (0.0) | 0 | 0 (0.0) | 0.0 |
SSC-AR, surgical site complication-associated readmission.
The risk score model (C-statistic =74.04%; 95% CI: 73.17–74.90%) using the development cohort suggested that, for each additional point in the risk score, the estimated probability of SSC-AR increased by 16% (OR, 1.16; P<0.0001) (Table 2, Figure 5).
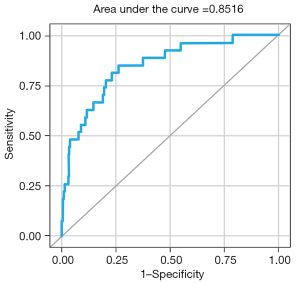
The validation process for the SSC-AR risk score was conducted using a random hold-out sample of 1,000 cases, of which 27 (2.7%) experienced SSC-ARs. These 1,000 surgery cases consisted of 80.8% primary fusion, 8.3% revision fusion, 0.9% primary non-fusion, and 10.0% revision non-fusion surgeries. The results of the validation demonstrated that the risk score maintained robust discriminatory capability (C-statistic =85.16%; 95% CI: 77.71–92.61%) (Figure 6), along with reasonable calibration (Pearson chi-square/DF =0.77).
When considering different score values as cutoffs to stratify surgery cases based on their SSC-AR risk, a medium risk score such as eight exhibited a sensitivity of 66.4% and specificity of 63.5%. This means that 66.4% of the total SSC-AR cases were identified in patients with a risk score ≥8, which also included 63.5% of the no-SSC-AR cases. A higher risk score, such as 20, was associated with higher specificity (99.2%) but lower sensitivity (4.0%) (Table 3). Using a cutoff score of eight captured a larger number of true SSC-ARs compared to a cutoff score of 20, but at the cost of a higher rate of false alarms.
DiscussionOther Section
In the present study, we examined the SSC-AR rate in a fusion-dominant population (87.5%), and its relationship with patient, facility, and procedure risk factors. By utilizing a more comprehensive definition of SSCs, we have expanded upon our previous SSI prediction model (39) to include a range of wound complications that occur following open spine surgeries. The complication rate (2.0%) identified through our database query is consistent with findings from other published studies. For instance, Piper et al., in their analysis of the National Surgical Quality Improvement Project (NSQIP) database, reported that 2.2% of spine surgery patients experienced at least one wound complication (11). Similarly, Weinstein et al. observed a wound infection rate of 1.9% in patients undergoing spinal surgery for lumbar degenerative scoliosis or spinal stenosis, disk prolapse, metastatic disease, and degenerative disk disease (19).
For this study, we developed and validated a point-of-care preoperative SSC-AR risk scoring system based on data from 158,664 cases of spine surgery which is the largest reported study population on this topic. A reduced model with comparable calibration and accuracy to the 37-variable full model, utilizing 19 clinical predictors, allows for less cumbersome utilization and increased applicability to a surgeon’s practice. The results of our model revealed that patients undergoing spine surgery with a risk score of ≥12 accounted for 13.6% of all surgeries but represented 37.6% of all SSC-ARs (Table 3). Such risk score cutoff levels, characterized by a high density of unplanned readmissions, can serve as clinical indicators for employing more aggressive measures, such as implementing advanced incisional management strategies (40,41), to reduce the occurrence of SSCs and ultimately bolster patient safety and surgical outcomes.
The predictors included in our reduced model align with risk factors reported in the existing literature. Previous studies have indicated that conditions such as hypertension, bleeding disorders, anemias, coagulation deficiencies, peripheral vascular disease, diabetes, chronic pulmonary disease, congestive heart failure, neurological disorders, rheumatoid arthritis, obesity, renal failure, cancer, liver disease, paralysis, drug abuse, alcohol abuse, and tobacco use, along with emergency cases, longer operating times (≥180 minutes), and revision surgeries, are associated with a higher rate of postoperative complications or unplanned readmissions following spine surgeries (5,28,30,32). Additional evidence supports that Charlson Comorbidity Index score is a significant predictor of readmission after orthopedic surgery (42), and pre-existing dementia and female gender are associated with an increased risk and burden of complications following spine surgery (11,43).
Predictive analytics aims to combat growing healthcare costs and enhance the value of the healthcare system. The ACS-SRC (22) and the SURPAS (23,24) derived from the ACS-NSQIP database, are considered gold standards for estimating postoperative complications across various surgical populations (26,44,45). However, such calculators, which rely on input variables and laboratory data, may have limited utility in emergency settings. A comparative study has even argued that both calculators overestimate readmission risk in higher-risk emergency patients (26). Universal calculators like ACS-SRC or SURPAS may fail to capture important factors specifically relevant to spine procedures and may not accurately predict spine surgery outcomes (7). Consequently, there has been a growing effort to develop predictive tools that are specific to diseases and surgical procedures in the field of spine surgery (46,47).
In a collaborative endeavor to improve outcomes in spine care, the American Association of Neurological Surgeons and the American Academy of Orthopaedic Surgeons established the first orthopedic/neurologic spine registry, known as the American Spine Registry (48). This registry facilitates the participation of all spine surgeons in the US and provides a shared platform for collecting procedural data, postoperative data, and patient-reported outcome measurements. Predictive analytics in spine surgery are still in the beginning stages and have employed techniques such as machine learning and statistical modeling. Within the realm of adult spinal deformity surgery, predictive modeling has been utilized to forecast patient-reported outcomes, hospital length of stay, blood transfusion requirements, pseudoarthrosis, complications, and costs (30,49-52). The European Spine Study Group has developed the Adult Deformity Surgery Complexity Index (ADSCI) to quantify the invasiveness of posterior adult spinal deformity surgery and identify patients who may be at risk of postoperative complications (53). Predictive models have also been employed to predict patient satisfaction following decompression for lumbar stenosis, functional outcomes after surgery for cervical spondylotic myelopathy and recurrent lumbar disk herniation (54-57), and outcomes following elective degenerative spine surgery (47).
The risk score model we present in this study, developed specifically for assessing the preoperative risk of SSC-AR in open spine surgery, particularly fusion procedures, is the first tool of its kind. Furthermore, we validated this model externally using a large healthcare database. This tool has the potential to improve workflow by enabling efficient, timely, and accurate risk stratification based on readily available clinical information. It allows practitioners to obtain a bedside risk profile for a patient’s likelihood of unplanned readmission following open spine surgery, facilitating the customization of perioperative risk management strategies to individualized patient risks. Implementing this risk score model into clinical practice may assist surgeons in identifying high-risk patients for which counseling and perioperative optimization protocols are needed to reduce the risk of experiencing costly complications and avoid unplanned readmissions. As these complications are ultimately being linked to quality metrics that determine prospective payments, our model may contribute to value-based care by guiding decision-making regarding risk modification for open spine procedures.
Table 3 illustrates how prediction would vary for each 2-point increase in cutoff score as a reference, providing surgeons with a reference to determine the levels that best suit their requirements. For instance, applying a 10-point cutoff would have enabled 23% of the surgeries to adopt preoperative SSC intervention targeting around 52% of unplanned readmissions. Developing an economic model could inform future studies on the cutoff levels of the risk score that yield positive returns for surgeons and/or health systems, considering the costs of intervention, SSC-AR rate, and readmission cost.
It is important to acknowledge that no predictive model can account for all risk factors, and every model has its limitations. The present model, for instance, did not consider the complete range of patient pathologies or specific distinctions in comorbidities. We had limited knowledge about the severity of comorbidities for each patient, such as duration, acute/chronic status, controllability. Likewise, information was unavailable to determine the purpose or history of spine surgery. We categorized the spine surgeries by screening each of the documented ICD-10 procedures to determine if a surgery had decisive codes for fusion or revision (e.g., removal of an existing internal fixation device). Surgeries with no defining procedures of fusion or revision were assigned to non-fusion or primary categories. Consequently, our study provides a more conservative estimate of the impact of surgical categories on SSC-AR. Surgery cases with SSCs that did not incur a readmission, or with readmissions not attributable to SSCs, were classified as the “no SSC-AR” group. Therefore, this study was modeling for the severe SSCs that had a costly consequence. Owing to the lack of laboratory data for the majority of the study population, we were unable to identify preoperative infections or infectious surgeries to enhance the refinement of our model. Additionally, the prediction tool we developed and validated was based on a population consisting of nearly 90% spinal fusions. Future research is needed to examine how well the risk score functions in more diverse spine surgery populations.
ConclusionsOther Section
Risk scores for predicting health outcomes are quickly becoming powerful, essential tools to inform perioperative management. The ability to accurately forecast wound-related surgical complications based on preoperative factors is an important step toward reducing unplanned readmissions and improving value-based care in open spine surgery. We developed and externally validated a point-of-care predictive tool for assessing SSC-AR risk in open spine surgery. The resulting risk score, derived from easily obtainable clinical information, holds significant potential as an effective assessment tool for risk stratification in preoperative settings when open spine surgery is considered.
AcknowledgmentsOther Section
Funding: None.
FootnoteOther Section
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-23-89/rc
Data Sharing Statement: Available at https://jss.amegroups.com/article/view/10.21037/jss-23-89/dss
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-23-89/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-23-89/coif). K.B.M. is a paid consultant for 3M Health Care. He has personally received payment/honoraria for his assistance in writing this manuscript. Y.H., K.B., and L.P.G. are employees of 3M. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study utilized deidentified data in accordance with 45 CFR 164.506(d)(2)(ii)(B), employing the “Expert Determination” method. As a result, it was exempt from institutional review board review and the requirement for informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Deyo RA, Mirza SK, Martin BI, et al. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA 2010;303:1259-65. [Crossref] [PubMed]
- Goz V, Weinreb JH, McCarthy I, et al. Perioperative complications and mortality after spinal fusions: analysis of trends and risk factors. Spine (Phila Pa 1976) 2013;38:1970-6. [Crossref] [PubMed]
- How Many Spinal Fusions are Performed Each Year in the United States? Burnaby: iData Research. [Updated 2018 May 25]. Available online: https://idataresearch.com/how-many-instrumented-spinal-fusions-are-performed-each-year-in-the-united-states/
- Lau D, Chan AK, Theologis AA, et al. Costs and readmission rates for the resection of primary and metastatic spinal tumors: a comparative analysis of 181 patients. J Neurosurg Spine 2016;25:366-78. [Crossref] [PubMed]
- Bekelis K, Desai A, Bakhoum SF, et al. A predictive model of complications after spine surgery: the National Surgical Quality Improvement Program (NSQIP) 2005-2010. Spine J 2014;14:1247-55. [Crossref] [PubMed]
- Potentially preventable readmissions classification system: methodology overview. Murray: 3M Health Information Systems. Contract No. GRP-139. 2012. Available online: https://multimedia.3m.com/mws/media/1684594O/3m-potentially-preventable-readmissions-methodology-overview.pdf
- Wang MC, Shivakoti M, Sparapani RA, et al. Thirty-day readmissions after elective spine surgery for degenerative conditions among US Medicare beneficiaries. Spine J 2012;12:902-11. [Crossref] [PubMed]
- Camacho JE, Kung JE, Thomson AE, et al. Retrospective Analysis of Causes and Risk Factors of 30-Day Readmission After Spine Surgery for Thoracolumbar Trauma. Global Spine J 2023;13:1558-65. [Crossref] [PubMed]
- McCormack RA, Hunter T, Ramos N, et al. An analysis of causes of readmission after spine surgery. Spine (Phila Pa 1976) 2012;37:1260-6. [Crossref] [PubMed]
- Higuera-Rueda CA, Emara AK, Nieves-Malloure Y, et al. The Effectiveness of Closed-Incision Negative-Pressure Therapy Versus Silver-Impregnated Dressings in Mitigating Surgical Site Complications in High-Risk Patients After Revision Knee Arthroplasty: The PROMISES Randomized Controlled Trial. J Arthroplasty 2021;36:S295-S302.e14.
- Piper KF, Tomlinson SB, Santangelo G, et al. Risk factors for wound complications following spine surgery. Surg Neurol Int 2017;8:269. [Crossref] [PubMed]
- Whitehouse JD, Friedman ND, Kirkland KB, et al. The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol 2002;23:183-9. [Crossref] [PubMed]
- Olsen MA, Butler AM, Willers DM, et al. Risk factors for surgical site infection after low transverse cesarean section. Infect Control Hosp Epidemiol 2008;29:477-84; discussion 485-6. [Crossref] [PubMed]
- Fang A, Hu SS, Endres N, et al. Risk factors for infection after spinal surgery. Spine (Phila Pa 1976) 2005;30:1460-5. [Crossref] [PubMed]
- Webb AL, Flagg RL, Fink AS. Reducing surgical site infections through a multidisciplinary computerized process for preoperative prophylactic antibiotic administration. Am J Surg 2006;192:663-8. [Crossref] [PubMed]
- De la Garza-Ramos R, Abt NB, Kerezoudis P, et al. Deep-wound and organ-space infection after surgery for degenerative spine disease: an analysis from 2006 to 2012. Neurol Res 2016;38:117-23. [Crossref] [PubMed]
- Patel H, Khoury H, Girgenti D, et al. Burden of Surgical Site Infections Associated with Select Spine Operations and Involvement of Staphylococcus aureus. Surg Infect (Larchmt) 2017;18:461-73. [Crossref] [PubMed]
- Carl HM, Ahmed AK, Abu-Bonsrah N, et al. Risk factors for wound-related reoperations in patients with metastatic spine tumor. J Neurosurg Spine 2018;28:663-8. [Crossref] [PubMed]
- Weinstein MA, McCabe JP, Cammisa FP Jr. Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. J Spinal Disord 2000;13:422-6. [Crossref] [PubMed]
- Nasser R, Yadla S, Maltenfort MG, et al. Complications in spine surgery. J Neurosurg Spine 2010;13:144-57. [Crossref] [PubMed]
- Bernatz JT, Anderson PA. Thirty-day readmission rates in spine surgery: systematic review and meta-analysis. Neurosurg Focus 2015;39:E7. [Crossref] [PubMed]
- Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013;217:833-42.e1-3.
- Meguid RA, Bronsert MR, Juarez-Colunga E, et al. Surgical Risk Preoperative Assessment System (SURPAS): I. Parsimonious, Clinically Meaningful Groups of Postoperative Complications by Factor Analysis. Ann Surg 2016;263:1042-8. [Crossref] [PubMed]
- Meguid RA, Bronsert MR, Juarez-Colunga E, et al. Surgical Risk Preoperative Assessment System (SURPAS): II. Parsimonious Risk Models for Postoperative Adverse Outcomes Addressing Need for Laboratory Variables and Surgeon Specialty-specific Models. Ann Surg 2016;264:10-22. [Crossref] [PubMed]
- Meguid RA, Bronsert MR, Juarez-Colunga E, et al. Surgical Risk Preoperative Assessment System (SURPAS): III. Accurate Preoperative Prediction of 8 Adverse Outcomes Using 8 Predictor Variables. Ann Surg 2016;264:23-31. [Crossref] [PubMed]
- Rozeboom PD, Bronsert MR, Velopulos CG, et al. A comparison of the new, parsimonious tool Surgical Risk Preoperative Assessment System (SURPAS) to the American College of Surgeons (ACS) risk calculator in emergency surgery. Surgery 2020;168:1152-9. [Crossref] [PubMed]
- PINC AITM Healthcare Data Special Release: COVID-19. Available online: https://offers.premierinc.com/rs/381-NBB-525/images/PHD_COVID-19_White_Paper.pdf
- Bernstein DN, Thirukumaran C, Saleh A, et al. Complications and Readmission After Cervical Spine Surgery in Elderly Patients: An Analysis of 1786 Patients. World Neurosurg 2017;103:859-868.e8. [Crossref] [PubMed]
- Glassman SD, Hamill CL, Bridwell KH, et al. The impact of perioperative complications on clinical outcome in adult deformity surgery. Spine (Phila Pa 1976) 2007;32:2764-70. [Crossref] [PubMed]
- Scheer JK, Smith JS, Schwab F, et al. Development of a preoperative predictive model for major complications following adult spinal deformity surgery. J Neurosurg Spine 2017;26:736-43. [Crossref] [PubMed]
- Corey KM, Kashyap S, Lorenzi E, et al. Development and validation of machine learning models to identify high-risk surgical patients using automatically curated electronic health record data (Pythia): A retrospective, single-site study. PLoS Med 2018;15:e1002701. [Crossref] [PubMed]
- Lee MJ, Cizik AM, Hamilton D, et al. Predicting medical complications after spine surgery: a validated model using a prospective surgical registry. Spine J 2014;14:291-9. [Crossref] [PubMed]
- Mirza SK, Deyo RA, Heagerty PJ, et al. Development of an index to characterize the "invasiveness" of spine surgery: validation by comparison to blood loss and operative time. Spine (Phila Pa 1976) 2008;33:2651-61; discussion 2662. [Crossref] [PubMed]
- Cizik AM, Lee MJ, Martin BI, et al. Using the spine surgical invasiveness index to identify risk of surgical site infection: a multivariate analysis. J Bone Joint Surg Am 2012;94:335-42. [Crossref] [PubMed]
- Smith JS, Shaffrey CI, Sansur CA, et al. Rates of infection after spine surgery based on 108,419 procedures: a report from the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976) 2011;36:556-63. [Crossref] [PubMed]
- Everhart JS, Andridge RR, Scharschmidt TJ, et al. Development and Validation of a Preoperative Surgical Site Infection Risk Score for Primary or Revision Knee and Hip Arthroplasty. J Bone Joint Surg Am 2016;98:1522-32. [Crossref] [PubMed]
- The GENMOD Procedure. In: SAS/STAT User's Guide: Version 8, Volume 2. Cary: SAS Institute Incorporated. Available online: https://support.sas.com/documentation/onlinedoc/stat/141/genmod.pdf
- Anderson DR, Burnham KP, White GC. Comparison of Akaike information criterion and consistent Akaike information criterion for model selection and statistical inference from capture-recapture studies. J Appl Stat 1998;25:263-82.
- Mueller KB, Hou Y, Beach K, et al. Development and validation of a point-of-care clinical risk score to predict surgical site infection following open spinal fusion. N Am Spine Soc J 2023;13:100196. [Crossref] [PubMed]
- Mueller KB, Sastry RA. The Importance of Incisional Management Strategies to Optimize Outcomes in Spine Surgery. World Neurosurg 2021;152:233-4. [Crossref] [PubMed]
- Mueller KB, D'Antuono M, Patel N, et al. Effect of Incisional Negative Pressure Wound Therapy vs Standard Wound Dressing on the Development of Surgical Site Infection after Spinal Surgery: A Prospective Observational Study. Neurosurgery 2021;88:E445-51. [Crossref] [PubMed]
- Voskuijl T, Hageman M, Ring D. Higher Charlson Comorbidity Index Scores are associated with readmission after orthopaedic surgery. Clin Orthop Relat Res 2014;472:1638-44. [Crossref] [PubMed]
- Kassahun WT. The effects of pre-existing dementia on surgical outcomes in emergent and nonemergent general surgical procedures: assessing differences in surgical risk with dementia. BMC Geriatr 2018;18:153. [Crossref] [PubMed]
- Havens JM, Columbus AB, Seshadri AJ, et al. Risk stratification tools in emergency general surgery. Trauma Surg Acute Care Open 2018;3:e000160. [Crossref] [PubMed]
- Khaneki S, Bronsert MR, Henderson WG, et al. Comparison of accuracy of prediction of postoperative mortality and morbidity between a new, parsimonious risk calculator (SURPAS) and the ACS Surgical Risk Calculator. Am J Surg 2020;219:1065-72. [Crossref] [PubMed]
- Lehner K, Ehresman J, Pennington Z, et al. Narrative Review of Predictive Analytics of Patient-Reported Outcomes in Adult Spinal Deformity Surgery. Global Spine J 2021;11:89S-95S. [Crossref] [PubMed]
- Lubelski D, Hersh A, Azad TD, et al. Prediction Models in Degenerative Spine Surgery: A Systematic Review. Global Spine J 2021;11:79S-88S. [Crossref] [PubMed]
- About the American Spine Registry: FAQS. Rosemont: American Spine Registry. [Updated 2022 Jul 29]. Available online: https://www.americanspineregistry.org/about-the-american-spine-registry/faqs/
- Scheer JK, Osorio JA, Smith JS, et al. Development of Validated Computer-based Preoperative Predictive Model for Proximal Junction Failure (PJF) or Clinically Significant PJK With 86% Accuracy Based on 510 ASD Patients With 2-year Follow-up. Spine (Phila Pa 1976) 2016;41:E1328-35. [Crossref] [PubMed]
- Safaee MM, Scheer JK, Ailon T, et al. Predictive Modeling of Length of Hospital Stay Following Adult Spinal Deformity Correction: Analysis of 653 Patients with an Accuracy of 75% within 2 Days. World Neurosurg 2018;115:e422-7. [Crossref] [PubMed]
- Durand WM, DePasse JM, Daniels AH. Predictive Modeling for Blood Transfusion After Adult Spinal Deformity Surgery: A Tree-Based Machine Learning Approach. Spine (Phila Pa 1976) 2018;43:1058-66. [Crossref] [PubMed]
- Ames CP, Smith JS, Gum JL, et al. Utilization of Predictive Modeling to Determine Episode of Care Costs and to Accurately Identify Catastrophic Cost Nonwarranty Outlier Patients in Adult Spinal Deformity Surgery: A Step Toward Bundled Payments and Risk Sharing. Spine (Phila Pa 1976) 2020;45:E252-65. [Crossref] [PubMed]
- Pellisé F, Vila-Casademunt A, Núñez-Pereira S, et al. The Adult Deformity Surgery Complexity Index (ADSCI): a valid tool to quantify the complexity of posterior adult spinal deformity surgery and predict postoperative complications. Spine J 2018;18:216-25. [Crossref] [PubMed]
- Azimi P, Benzel EC, Shahzadi S, et al. Use of artificial neural networks to predict surgical satisfaction in patients with lumbar spinal canal stenosis: clinical article. J Neurosurg Spine 2014;20:300-5. [Crossref] [PubMed]
- Shamim MS, Enam SA, Qidwai U. Fuzzy Logic in neurosurgery: predicting poor outcomes after lumbar disk surgery in 501 consecutive patients. Surg Neurol 2009;72:565-72; discussion 572. [Crossref] [PubMed]
- Hoffman H, Lee SI, Garst JH, et al. Use of multivariate linear regression and support vector regression to predict functional outcome after surgery for cervical spondylotic myelopathy. J Clin Neurosci 2015;22:1444-9. [Crossref] [PubMed]
- Azimi P, Mohammadi HR, Benzel EC, et al. Use of artificial neural networks to predict recurrent lumbar disk herniation. J Spinal Disord Tech 2015;28:E161-5. [Crossref] [PubMed]