
Enhanced recovery after elective spinal surgery: an Australian pilot study
Highlight box
Key findings
• The study demonstrates the feasibility and safety of implementing enhanced recovery after surgery (ERAS) principles in decompressive spinal surgeries without compromising on patient outcomes up to 6 months post-surgery.
• Being of non-English speaking background and from home alone were predictors of failure of same-day discharge in our cohort.
What is known and what is new?
• ERAS principles across various surgical specialties have delivered patient optimization through their surgical journey resulting in improvement in patient and health economic outcomes.
• This study is a first of its kind in Australia and gives insight into predictors of failure in this cohort.
What is the implication, and what should change now?
• ERAS methodology for spinal surgery should be embraced across Australia to reduce burden on hospitals without increasing patient complications.
IntroductionOther Section
Since its inception in the 1990s (1), enhanced recovery after surgery (ERAS) protocols have emerged to reduce the physiological stress of surgery to improve patient and health economic outcomes. It does so by applying multimodal and interdisciplinary perioperative care to reduce physiological stress of surgery and maintain homeostasis (2). Coordination and engagement among multiple teams (anaesthetic, surgical, allied health staff, nursing) and patients is paramount to deliver a unified and iterative approach to the patient’s journey at a high level of quality. As such, the ERASÒ society (www.erassociety.org) has developed evidence driven guidelines for the successful implementation and audit of these perioperative pathways in various specialties. Although its roots began in colorectal surgery multiple surgical fields such as cardiothoracic (3), gynaecology (4) and orthopaedic joint surgery (5) have applied speciality-specific protocols with published successes over the last decade. More recently its implementation and assessment has been adopted to spine surgery across the world, but in Australia it still remains in its infancy.
Considering the recent coronavirus disease 2019 (COVID-19) pandemic, its crippling burden on our hospitals and generation of a massive elective case backlog (6,7), strategies to reduce inpatient admissions and cancelled elective surgeries due to bed and staff shortages are paramount. These strategies also need to address minimising the physiological impact of surgery on our aging global population with an increasing burden of disease caused by spinal pathologies (8). The ERAS paradigm aims to achieve this by minimising the physiological, psychological and social stress that surgery places on each patient, thus reducing LOS and hospitalisation costs without increasing complications or readmissions (9-13). This study aims to provide early experience at a single institution in Australia in implementing ERAS protocols in simple spinal surgery (1- or 2-level laminectomy, discectomy or decompression), with a focus on identifying factors that would predict failure. It aims to serve as a steppingstone for implementation, evaluation, and iterative improvement of ERAS protocols in spine. We present this article in accordance with the STROBE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-23-115/rc).
MethodsOther Section
Study design
This study is a prospective cohort analysis of suitable consecutive patients, to demonstrate the feasibility of implementing and actioning an ERAS protocol for patients undergoing simple spine surgery by a single neurosurgeon (Y.L.) at Westmead Public and Private Hospitals. The protocol was implemented by March 2021 with the first patient being enrolled in that month and the final patient in May 2023. Inclusion and exclusion criteria have been detailed in Table 1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). It was deemed to be of negligible risk according to the National Statement on Ethical Conduct in Human Research (14) and granted an exemption by the Western Sydney Local Health District Human Research Ethics Committee with individual consents waived.
Table 1
| Inclusion criteria |
| Age: 18–80 years |
| 1- or 2-level cervical or lumbar decompression surgery (microdiscectomy, laminectomy, foraminotomy) |
| Ability to understand and participate in the program |
| Exclusion criteria |
| Deemed not suitable by anaesthetic/surgical teams |
| Patients with neurological deficit requiring inpatient rehabilitation |
ERAS protocol
The last few years have seen an increase in publications for implementation of ERAS protocols in the neurosurgical arena (15-22). The ERASÒ society has also recently published a consensus statement for perioperative care in lumbar spinal fusion requiring a multidisciplinary team including surgeons, anaesthesiologists, nursing staff, physiotherapists, social services and hospital administration for successful implementation and audit (23). Our ERAS protocol has taken inspiration from these publications and guidelines and is divided into five distinct chronological periods detailing a spinal patient’s operative journey (Figure 1). The components of each of these will be reviewed here.
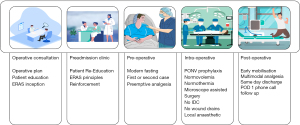
Once a patient meets the inclusion criteria, they undergo a detailed discussion and informed consent regarding their planned operative procedure during their operative consultation with a medical member from the neurosurgical team. At this time, they are also introduced to the idea of ERAS principles—minimally invasive surgery, immediate mobilisation with multimodal analgesics and same day discharge. This also allows for the patient to be pre-optimised from a physical and functional status by assessing, educating and referring them (if indicated) for better diabetes control, nutritional supplementation, smoking cessation and narcotic/alcohol use.
During the preadmission clinic, these principles are reiterated, and their expectations are set to encourage their own initiative and motivation for recovery postoperatively. Preoperatively, the patients are advised to fast as per modern fasting guidelines (24) and receive preemptive analgesia (200 mg celecoxib, 75 mg pregabalin and 1 g of paracetmol) (25,26) when they are checked in on the morning of their surgery.
The surgery is performed under general anaesthesia with infiltration of a weight based maximal dose of bupivacaine with adrenaline (0.25%)—half delivered pre-incision and half at wound closure. Intraoperatively they receive 1 mg/kg oxycodone, 10–20 mmol Magnesium sulfate (27) and 2 mcg/kg clonidine (28) for intraoperative comfort and opioid sparing post-operative analgesia. The surgery is performed with the assistance of an operating microscope employing minimally invasive techniques such as unilateral laminectomy for bilateral decompression. Foley catheters or wound drains are not used. Goal directed fluid management and convective warming devices are adopted. Eight mg of dexamethasone was administered at time of induction for post operative nausea and vomiting (PONV) prophylaxis.
Post-operatively, opioid sparing multimodal analgesia (regular paracetamol, regular celecoxib with tapentadol PRN as required) is provided as a script at time of discharge. The patient is assessed by the physiotherapy team in post-operative recovery and discharged within 4 hours post-procedure. After discharge the patient was contacted postoperative day 1 (POD 1) via phone call and then reviewed POD 5 in clinic (if local). They then had subsequent routine follow-up at 6 weeks post-operatively.
Study parameters
Primary outcomes included length of stay (LOS), patient reported outcomes (PROs) and complications (readmission within 30 days, post-operative wound infection or any other adverse event that could be attributed to surgery or same day discharge). PROs were measured using Numeric Rating Score (NRS) in conjunction with Oswestry Disability Index (ODI) and Neck Disability Index (NDI) for patients undergoing lumbar surgery and cervical surgery respectively. These questionnaires were filled out by the patient at their pre-operative, 6-week post-operative and 6-month post-operative follow-up appointments. Patient demographics, medical comorbidities and operative parameters were also analysed specifically looking for predictors of failure and/or complications.
Statistical analysis
Descriptive statistics were used to summarise the characteristics of the enrolled cohort. Continuous variables were described using mean and standard deviation and categorical variables were described using counts and percentages. Associations with failed same day discharge were measured using logistic regression for continuous dependent variables. Fisher’s exact test was used for categorical dependent variables with any cell counts less than five, otherwise the Chi square test was used. Odds ratios (ORs) were calculated with 95% confidence intervals. A two-tailed alpha of 0.05 was used as the threshold for statistical significance. All analyses were conducted using R version 4.3.0.
ResultsOther Section
A total of 52 patients were enrolled in this pilot study between March 2021 to May 2023. Patient demographics along with social and procedural specifics are summarised in Table 2.
Table 2
| Characteristic | Value |
|---|---|
| Demographic | |
| Age, years, mean ± SD | 49.2±14.3 |
| Female sex, n [%] | 18 [35] |
| BMI >30 kg/m2, n [%] | 26 [50] |
| Preoperative narcotic use, n [%] | 11 [21] |
| Current smoker, n [%] | 18 [35] |
| ASA, n [%] | |
| 1 | 8 [15] |
| 2 | 36 [69] |
| 3 | 8 [15] |
| Blood thinners, n [%] | 5 [10] |
| Psychiatric history, n [%] | 10 [19] |
| Social, n [%] | |
| Non-English speaking background | 9 [17] |
| At home alone | 5 [10] |
| Rural residence | 7 [13] |
| Procedural, n [%] | |
| Pre-operative weakness | 13 [25] |
| Operation type | |
| Cervical | 7 [13] |
| Lumbar laminectomy | 5 [10] |
| Lumbar LRD | 11 [21] |
| Lumbar microdiscectomy | 22 [42] |
| Lumbar ULBD | 7 [13] |
| Multilevel | 3 [6] |
SD, standard deviation; BMI, body mass index; ASA, American Society of Anesthesiologists; LRD, lateral recess decompression; ULBD, unilateral laminectomy and bilateral decompression.
Nine of the 52 patients (17.3%) were not successfully discharged on the same day of their operation, with 7 discharging POD 1 and the remaining 2 POD 2. The reasons for these patients’ inability to be discharged on the same day are outlined in Table 3 in chronological order.
Table 3
| Case | Reason(s) for failure |
|---|---|
| 1 | Last case on operating list, day-only ward closed and unable to accommodate patient |
| 3 | Communication breakdown with no transport home |
| 13 | Anaesthetist of the day felt strongly that patient needed inpatient monitoring |
| 23 | Asymptomatic post-operative hypotension with non-specific ECG changes |
| 25 | Rural domicile with no local accommodation |
| 28 | Post-operative surgical wound pain |
| 33 | Asymptomatic post-operative hypotension |
| 41 | Post-operative desaturation due to undiagnosed OSA |
| 43 | Post-operative nausea and vomiting, rural domicile |
ECG, electrocardiogram; OSA, obstructive sleep apnoea.
The rate of complications in this cohort was 7.7% (4 out of 52) patients. These included 1 patient who had a durotomy, 1 with a wound haematoma, 1 with a superficial wound infection, and 1 presentation to the emergency department with recurrent radicular pain. The patients were all managed conservatively and did not require readmission. One patient had recurrent symptoms at their 12-month follow-up and had a reoperation.
Pre-operative, 6-week post-operative and 6-month post-operative PROs are summarised in Table 4 and demonstrate an expected improvement in pain and functional scores that were sustained at their 6-month follow-up (P<0.0001).
Table 4
| Outcome | Pre-operatively | Post-operatively (6-weeks) | Post-operatively (6-months) |
|---|---|---|---|
| NRS for back or neck pain | 6.6±2.8 | 2.7±2.5 | 3.0±2.9 |
| NRS for leg or arm pain | 7.9±2.2 | 2.1±2.8 | 3.0±3.4 |
| ODI/NDI | 51±20 | 20±18 | 27±25 |
Data are presented as mean ± SD. PRO, patient reported outcome; NRS, Numeric Rating Score; ODI, Oswestry Disability Index; NDI, Neck Disability Index; SD, standard deviation.
Patient demographics and procedural specific factors were analysed as associated causes predicting failure of the ERAS protocol and are summarised in Table 5. Patient demographics, weight, pre-operative narcotic use, medical comorbidities, and procedure specific factors were not predictors of failure in this cohort of 52 patients. However, if the patient was of non-English speaking background (NESB) (OR =6.08, P=0.04) or was from home alone (OR =10.25, P=0.03), they were more likely to fail the ERAS protocol. Patients with multilevel surgeries, cervical surgeries and unilateral laminectomy and bilateral decompression (ULBD) procedures had no failures and thus ORs could not be calculated.
Table 5
| Characteristic | Success (n=43) | Failure (n=9) | OR (95% CI) | P value |
|---|---|---|---|---|
| Age, years, mean ± SD | 48.0±14.2 | 55.0±14.3 | 0.96 (0.91–1.02) | 0.18 |
| Female sex, n [%] | 14 [29] | 4 [44] | 1.66 (0.38–7.15) | 0.70 |
| BMI >30 kg/m2, n [%] | 21 [49] | 5 [56] | 1.31 (0.31–5.55) | 1.00 |
| Narcotic use, n [%] | 10 [23] | 1 [11] | 0.41 (0.05–3.71) | 0.66 |
| Current smoker, n [%] | 15 [35] | 4 [44] | 1.49 (0.35–6.41) | 0.71 |
| ASA, n [%] | >0.99 | |||
| 1 (reference) | 7 [16] | 1 [11] | – | |
| 2 | 29 [67] | 7 [78] | 1.69 (0.18–16.06) | |
| 3 | 7 [16] | 1 [11] | 1.00 (0.05–19.36) | |
| Blood thinners, n [%] | 3 [7] | 2 [22] | 3.81 (0.54–27.08) | 0.20 |
| Psychiatric history, n [%] | 8 [19] | 2 [22] | 1.25 (0.22–7.19) | >0.99 |
| NESB, n [%] | 5 [12] | 4 [44] | 6.08 (1.21–30.47) | 0.04 |
| At home alone, n [%] | 2 [5] | 3 [33] | 10.25 (1.41–74.51) | 0.03 |
| Regional or rural residence, n [%] | 5 [12] | 2 [22] | 2.17 (0.35–13.50) | 0.59 |
| Pre-operative weakness, n [%] | 9 [21] | 4 [44] | 3.02 (0.67–13.63) | 0.20 |
| Region, n [%] | 0.33 | |||
| Cervical (reference) | 7 [16] | 0 [0] | – | |
| Lumbar | 36 [84] | 9 [100] | * | |
| Type of lumbar operation, n [%] | 0.26 | |||
| Laminectomy (reference) | 3 [7] | 2 [22] | – | |
| Midline-preserving procedures | 33 [77] | 7 [78] | 0.32 (0.04–2.27) | |
| ULBD | 7 [16] | 0 [0] | * | 0.15 |
| Multilevel, n [%] | 3 [7] | 0 [0] | * | >0.99 |
*, OR calculation not possible due to 0 failures. OR, odds ratio; CI, confidence interval; SD, standard deviation; BMI, body mass index; ASA, American Society of Anesthesiologists; LRD, lateral recess decompression; NESB, non-English speaking background; ULBD, unilateral laminectomy and bilateral decompression; ERAS, enhanced recovery after surgery.
DiscussionOther Section
Over the last two decades, various authors have published results in relation to outpatient or same-day discharge operative interventions for degenerative lumbar pathologies without increasing 30-day readmission rates or complications and with significant improvement in pain scores (17,29-31). Although not strictly within an overarching ERAS framework, these studies have proven to be stepping stones to the first proposal of the application of ERAS in major spine surgery by Wainwright et al. (15). Since then, ERAS has gained significant momentum into populations undergoing elective spine surgery (16,18-20,32,33). We report the clinical outcomes of the first prospective cohort study from Australia in which an ERAS protocol was implemented for a population of patients that underwent decompressive elective spine surgery and search for predictors of its failure.
Our study demonstrates a success rate of 82.7% (43 out of 52 patients) for same day discharge with no readmissions within 30 days from surgery. The PROs for NRS also show improvements from 6.6 to 2.7 for back/neck pain and 7.9 to 3.0 for leg/arm pain at 6 months post-surgery. The ODI/NDI results were similarly improved from 51 preoperatively to 27 at the 6-month follow-up. These numbers are comparable to other similar studies looking at simple spinal decompressions performed under ERAS principles (16,19,20). Post-operative complication rates in lumbar laminectomies range from 0–15.8% with dural tears, post-operative infection and pain being the most prominent concerns (34). Our complication rate of 7.7%, with none requiring inpatient care or reoperation is comparable to these published results both performed as day cases or as inpatients (35). Overall, our outcomes, albeit in a small population sample demonstrate durability of the surgery performed under ERAS principles without compromising patient care.
Many factors including patient comorbidities, post-operative pain, complications and post operative fear of movement affect LOS in patients undergoing spinal surgery. Through the ERAS framework most of these factors can be addressed at various points in the patient’s surgical journey. The inception of early mobilisation from the operative consultation stage, reinforced along subsequent contact points helps in setting expectations and motivating them to enhance their recovery immediately after surgery. This motivation is then reinforced by perioperative multimodal analgesia, minimally invasive surgical techniques, no usage of Foley catheter or wound drains, to minimise the physiological impact of surgery and providing an ideal environment for their recovery.
Analysing various factors that increase likelihood of failure of same-day discharge, we found that being of NESB (OR =6.08, P=0.04) or from home alone (OR =10.25, P=0.03) were the only statistically significant contributors in patients requiring inpatient admission. Although all NESB patients had consultations with healthcare approved telephone interpreter services throughout their ERAS journey, they are more likely to feel like a burden to the medical team and are often embarrassed to admit their inability to understand specifics (36). Culturally, these patients may believe in traditional concepts that longer LOS means better care and recovery (37)—an ideology that may be hard to change over a period of two pre-operative consultations. This ultimately limits these patients’ ability to participate in shared decision making and result in non-adherence to the ERAS protocol compared to English-speaking patients. We propose the use of in-person interpreter services in the presence of English-speaking family members or friends to better disseminate the ERAS principles in these patients to reduce their likelihood of failure.
There is ample evidence in literature that patients with disparities among social determinants of health can impact overall wellbeing and surgical outcomes (38,39). Being currently married, having a partner at home and generally having social connectivity with relatives and friends is associated with shorter LOS, 30-day representations and readmissions (39). Our study has also demonstrated that having no family or friends that are able to pick a patient up when they are ready for discharge and stay with them at home is the highest predictor of failed same-day discharge. Although not including patients from home alone is an option to improve ERAS success, other options such as the use of medihotels for patients with no transport home or overnight assistance can also unburden hospitals from unnecessary social admissions.
Body mass index (BMI) >30 kg/m2 and age >65 years have been demonstrated as predictors of failure of ERAS, particularly in the field of colorectal surgery (40,41). In our small population cohort, neither of these factors reached statistical significance likely due to such small sample size, but also due to the direct impact BMI has on open or laparoscopic colorectal surgery.
Despite the use of minimally invasive surgery (MIS) techniques, certain spinal procedures can be more invasive than others—traditional laminectomies compared to ULBD; single level compared to multilevel. In our analysis, we were unable to demonstrate any relationship between the type of surgery and failure of same-day discharge, but this is likely attributable to small case numbers in each subset of included surgeries.
One of the key benefits of having an iterative ERAS protocol is its ability to adapt and improve over time and be flexible enough to accommodate various patient population demographics. For example, in our study, the first patient failed same-day discharge as the case was done later in the day; this resulted in the modification of the ERAS protocol for patients to be done either first or second on the elective operating list. Similarly, cases cancelled due to disagreement between anaesthetists regarding suitability for discharge on the same day resulted in ensuring patient review in pre-admission clinic by the same anaesthetist that would be involved on the day of surgery.
At its core, ERAS is about improving patient outcomes and speeding up patient recovery by optimising their surgical experience. As per the consensus guidelines by the ERASÒ society (23), 22 items have been identified for lumbar fusion looking at every aspect of a patient’s journey and promoting the patient as an active participant in their recovery and rehabilitation. This paradigm relies on multidisciplinary and collaborative care of various specialties involved in the patient’s surgical journey to engage in a standardised fashion. The incremental benefits of its various elements translate to better patient and health outcomes and demonstrated in multiple systematic reviews (9-13) and are confirmed albeit in a small population in our Australian first study.
There are, however, several limitations to this study. It is prospective cohort analysis which is primarily limited by its small sample size. Randomisation and blinding were not performed due to limited resources and single surgeon involvement, and this introduces a selection bias within our patient population. There were minor deviations from the multimodal opioid sparing analgesic regime depending on the anaesthetist involved which were not well documented and difficult to assess. This pilot study only included patients undergoing simple spinal decompressions using a targeted set of ERAS interventions. Although assessment of nutritional status and pre-operative nutritional optimization form part of ERASÒ society spinal fusion guidelines (23), due to resource and personnel limitations, these could not be incorporated into our implemented protocol and should be included in future iterations of the protocol. A larger study population with inclusion of more complex spinal surgeries (such as instrumented fusions and disc replacements) as well randomisation to eliminate selection bias should be considered in future studies.
Despite these limitations, this study demonstrates the safety and efficacy of our ERAS protocol and the feasibility of its implementation without significant overhead. Patient social factors play an important role in same day discharges for simple spinal surgeries and a formalised ERAS pathway allows for better surgical education for patients preoperatively. Such an ERAS protocol can be safely incorporated in other centres across Australia to help reduce burden of inpatient admissions from elective spine surgery whilst improving patient outcomes.
ConclusionsOther Section
There is strong emerging evidence to support the adoption of ERAS principles across spine surgery to improve LOS, complication rates, post-operative pain and functional outcomes. This is particularly important with increasing demand for spine surgery and increasing burden on our hospital admissions and staff, recently brought to light with the public health crisis during the COVID-19 pandemic. Our study shows that the adoption of such a comprehensive ERAS program in spine surgery is beneficial and simple to apply. It demonstrates the special attention that needs to be placed on social factors and patient understanding, especially in a multicultural and socio-economic diverse population like Australia, to facilitate its success.
AcknowledgmentsOther Section
We would like to thank the various members of the multidisciplinary teams at Westmead Hospital including nursing staff, physiotherapists, anesthetists and clerical staff who contributed to patient care and data acquisition.
Funding: None.
FootnoteOther Section
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-23-115/rc
Data Sharing Statement: Available at https://jss.amegroups.com/article/view/10.21037/jss-23-115/dss
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-23-115/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-23-115/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). It was deemed to be of negligible risk according to the National Statement on Ethical Conduct in Human Research and granted an exemption by the Western Sydney Local Health District Human Research Ethics Committee with individual consents waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 1997;78:606-17. [Crossref] [PubMed]
- Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg 2017;152:292-8. [Crossref] [PubMed]
- Laohathai S, Sadad Z, Suvarnakich K, et al. Efficacy of the Enhanced Recovery After Surgery program for thoracic surgery in a developing country. Indian J Thorac Cardiovasc Surg 2023;39:476-83. [Crossref] [PubMed]
- Bogani G, Sarpietro G, Ferrandina G, et al. Enhanced recovery after surgery (ERAS) in gynecology oncology. Eur J Surg Oncol 2021;47:952-9. [Crossref] [PubMed]
- Zhao X, Chen L, Huang F, et al. Enhanced Recovery after Surgery in patients undergoing total joint arthroplasty: A retrospective study. Pak J Med Sci 2023;39:644-9. [Crossref] [PubMed]
- Macdonald N, Clements C, Sobti A, et al. Tackling the elective case backlog generated by Covid-19: the scale of the problem and solutions. J Public Health (Oxf) 2020;42:712-6. [Crossref] [PubMed]
- Mills ES, Mertz K, Faye E, et al. The Effect of COVID-19 on Spine Surgery. Global Spine J 2023; Epub ahead of print. [Crossref]
- Fehlings MG, Tetreault L, Nater A, et al. The Aging of the Global Population: The Changing Epidemiology of Disease and Spinal Disorders. Neurosurgery 2015;77:S1-5. [Crossref] [PubMed]
- Dietz N, Sharma M, Adams S, et al. Enhanced Recovery After Surgery (ERAS) for Spine Surgery: A Systematic Review. World Neurosurg 2019;130:415-26. [Crossref] [PubMed]
- Elsarrag M, Soldozy S, Patel P, et al. Enhanced recovery after spine surgery: a systematic review. Neurosurg Focus 2019;46:E3. [Crossref] [PubMed]
- Pennington Z, Cottrill E, Lubelski D, et al. Systematic review and meta-analysis of the clinical utility of Enhanced Recovery After Surgery pathways in adult spine surgery. J Neurosurg Spine 2020;34:325-47. [Crossref] [PubMed]
- Tong Y, Fernandez L, Bendo JA, et al. Enhanced Recovery After Surgery Trends in Adult Spine Surgery: A Systematic Review. Int J Spine Surg 2020;14:623-40. [Crossref] [PubMed]
- Zaed I, Bossi B, Ganau M, et al. Current state of benefits of Enhanced Recovery After Surgery (ERAS) in spinal surgeries: A systematic review of the literature. Neurochirurgie 2022;68:61-8. [Crossref] [PubMed]
- National Statement on Ethical Conduct in Human Research. Canberra: National Health and Medical Research Council; 2023. Report No.: E72C. Available online: www.nhmrc.gov.au/about-us/publications/national-statement-ethical-conduct-human-research-2023
- Wainwright TW, Wang MY, Immins T, et al. Enhanced recovery after surgery (ERAS)—Concepts, components, and application to spine surgery. Seminars Spine Surg 2018;30:104-10.
- Soffin EM, Vaishnav AS, Wetmore DS, et al. Design and Implementation of an Enhanced Recovery After Surgery (ERAS) Program for Minimally Invasive Lumbar Decompression Spine Surgery: Initial Experience. Spine (Phila Pa 1976) 2019;44:E561-70. [Crossref] [PubMed]
- Scanlon J, Richards B. Development of a same day laminectomy program. J Perianesth Nurs 2004;19:84-8. [Crossref] [PubMed]
- Lu Y, Long J, Leng X, et al. Enhanced recovery after microdiscectomy: reductions in opioid use, length of stay and cost. BMC Surg 2023;23:259. [Crossref] [PubMed]
- Tarıkçı Kılıç E, Demirbilek T, Naderi S. Does an enhanced recovery after surgery protocol change costs and outcomes of single-level lumbar microdiscectomy? Neurosurg Focus 2019;46:E10. [Crossref] [PubMed]
- Debono B, Sabatier P, Garnault V, et al. Outpatient Lumbar Microdiscectomy in France: From an Economic Imperative to a Clinical Standard-An Observational Study of 201 Cases. World Neurosurg 2017;106:891-7. [Crossref] [PubMed]
- Dangayach NS, Caridi J, Bederson J, et al. Enhanced Recovery After Neurosurgery: Paradigm Shift and Call to Arms. World Neurosurg 2017;100:683-5. [Crossref] [PubMed]
- Ali ZS, Ma TS, Ozturk AK, et al. Pre-optimization of spinal surgery patients: Development of a neurosurgical enhanced recovery after surgery (ERAS) protocol. Clin Neurol Neurosurg 2018;164:142-53. [Crossref] [PubMed]
- Debono B, Wainwright TW, Wang MY, et al. Consensus statement for perioperative care in lumbar spinal fusion: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Spine J 2021;21:729-52. [Crossref] [PubMed]
- Søreide E, Ljungqvist O. Modern preoperative fasting guidelines: a summary of the present recommendations and remaining questions. Best Pract Res Clin Anaesthesiol 2006;20:483-91. [Crossref] [PubMed]
- Kien NT, Geiger P, Van Chuong H, et al. Preemptive analgesia after lumbar spine surgery by pregabalin and celecoxib: a prospective study. Drug Des Devel Ther 2019;13:2145-52. [Crossref] [PubMed]
- Jiang HL, Huang S, Song J, et al. Preoperative use of pregabalin for acute pain in spine surgery: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96:e6129. [Crossref] [PubMed]
- Silva Filho SE, Sandes CS, Vieira JE, et al. Analgesic effect of magnesium sulfate during total intravenous anesthesia: randomized clinical study. Braz J Anesthesiol 2021;71:550-7. [Crossref] [PubMed]
- Savitha KS, Dhanpal R, Kothari AN. The Effect of Multimodal Analgesia on Intraoperative Morphine Requirement in Lumbar Spine Surgeries. Anesth Essays Res 2017;11:397-400. [Crossref] [PubMed]
- Chin KR, Coombs AV, Seale JA. Feasibility and patient-reported outcomes after outpatient single-level instrumented posterior lumbar interbody fusion in a surgery center: preliminary results in 16 patients. Spine (Phila Pa 1976) 2015;40:E36-42. [Crossref] [PubMed]
- Chin KR, Pencle FJ, Coombs AV, et al. Lateral Lumbar Interbody Fusion in Ambulatory Surgery Centers: Patient Selection and Outcome Measures Compared With an Inhospital Cohort. Spine (Phila Pa 1976) 2016;41:686-92. [Crossref] [PubMed]
- Eckman WW, Hester L, McMillen M. Same-day discharge after minimally invasive transforaminal lumbar interbody fusion: a series of 808 cases. Clin Orthop Relat Res 2014;472:1806-12. [Crossref] [PubMed]
- Soffin EM, Wetmore DS, Beckman JD, et al. Opioid-free anesthesia within an enhanced recovery after surgery pathway for minimally invasive lumbar spine surgery: a retrospective matched cohort study. Neurosurg Focus 2019;46:E8. [Crossref] [PubMed]
- Ali ZS, Flanders TM, Ozturk AK, et al. Enhanced recovery after elective spinal and peripheral nerve surgery: pilot study from a single institution. J Neurosurg Spine 2019; Epub ahead of print. [Crossref]
- Goacher E, Sanders MI, Ivanov M. Safety and feasibility of same-day discharge following lumbar decompression surgery: A systematic review. Brain Spine 2022;2:100888. [Crossref] [PubMed]
- German JW, Adamo MA, Hoppenot RG, et al. Perioperative results following lumbar discectomy: comparison of minimally invasive discectomy and standard microdiscectomy. Neurosurg Focus 2008;25:E20. [Crossref] [PubMed]
- Ngo-Metzger Q, Sorkin DH, Phillips RS, et al. Providing high-quality care for limited English proficient patients: the importance of language concordance and interpreter use. J Gen Intern Med 2007;22:324-30. [Crossref] [PubMed]
- Newsome F, McDonnell JM, Macken M, et al. Barriers to consent in spine surgery. Spine J 2022;22:1073-8. [Crossref] [PubMed]
- Laxton AW, Perrin RG. The relations between social support, life stress, and quality of life following spinal decompression surgery. Spinal Cord 2003;41:553-8. [Crossref] [PubMed]
- Holbert SE, Andersen K, Stone D, et al. Social Determinants of Health Influence Early Outcomes Following Lumbar Spine Surgery. Ochsner J 2022;22:299-306. [Crossref] [PubMed]
- Renz BW, Kasparek MS, Seeliger H, et al. The CR-POSSUM Risk Calculator Predicts Failure of Enhanced Recovery after Colorectal Surgery. Acta Chir Belg 2015;115:20-6.
- Zhang Y, Xin Y, Sun P, et al. Factors associated with failure of Enhanced Recovery After Surgery (ERAS) in colorectal and gastric surgery. Scand J Gastroenterol 2019;54:1124-31. [Crossref] [PubMed]


