Intraoperative fat embolism syndrome associated with implantation of titanium sacroiliac joint fusion implants: a report of two cases
Highlight box
Key findings
• Two patients experienced intraoperative fat embolism syndrome during sacroiliac joint fusion implant placement, one requiring cardiopulmonary resuscitation.
What is known and what is new?
• Fat embolism is a known, rare complication of spinal fusion surgery.
• No previous cases of fat embolism syndrome in sacroiliac fusion have been reported.
What is the implication, and what should change now?
• Spine surgeons should be aware of this rare but potentially fatal complication and warn their anesthesiology teams prior to implant placement.
Introduction
Degenerative kyphoscoliosis with other degenerative pathologies of the lumbar spine is a common problem, affecting 6–68% of the elderly population (1). For patients undergoing surgery to correct lumbar degenerative kyphoscoliosis, sacroiliac joint (SIJ) fusion has been proposed as an adjunct. This may be done if the patient has pre-existing SIJ dysfunction and/or to stabilize the SIJ in the face of long-construct fusions. Prophylactic fusion is a controversial topic that is currently being investigated. It is known that many patients undergoing lumbopelvic fusion develop post-operative SIJ pain (2-4). Historically, screws and plates were the traditional choice of instrumentation when SIJ fusion was required. More recently, the development of new implants and techniques offers alternative options for fixation and approach. These new implants provide excellent biomechanical stability and the opportunity to augment the fixation with bone graft within the device itself (5,6).
Intraoperative pulmonary fat embolism is a rare but known complication of spinal pedicle screw instrumentation (7-11) and cement augmentation (12-14). Data on this phenomenon is limited primarily to case reports, highlighting the infrequent nature of the problem. To our knowledge, no previous reports have documented the occurrence of fat embolism associated with implantation of a titanium SIJ fusion device. We report on two cases during which intraoperative fat embolism occurred immediately following placement of the implant. We present this article in accordance with the CARE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-23-87/rc).
Case presentation
Case 1
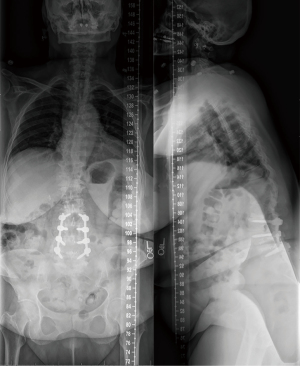
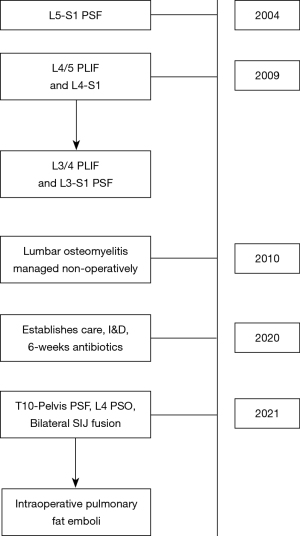
The patient was a 50-year-old female with a past medical history of ankylosing spondylitis, hypertension, and tobacco use, who presented to our clinic with a complicated surgical history. The patient had undergone previous L5–S1 posterior spinal fusion (PSF) 17 years prior, followed by adjacent level disease requiring extension posterior fusion to L4 and L4/L5 interbody fusion twelve years later. She subsequently developed adjacent level disease again in that same year and underwent extension posterior fusion to L3 and interbody fusion of L3/L4. After the last surgery, the patient developed L3–L4 osteomyelitis/diskitis that was managed non-operatively with multiple long-term courses of antibiotics and chronic oral antibiotic suppression. Once established with our surgical team, the patient underwent irrigation and debridement (I&D) and removal of the posterior instrumentation with retained interbody implants, followed by six weeks of intravenous antibiotics. Imaging at that time demonstrated significant loss of lumbar lordosis consistent with iatrogenic flatback syndrome (Figure 1). She had no clinical indications or imaging findings concerning for ongoing infection, and normal inflammatory markers. The patient demonstrated symptoms consistent with sagittal plane deformity with sagittal plane fatigue refractory to nonoperative management. After discussion with the patient, the decision was made to proceed with a T10-pelvis PSF with an L4 pedicle subtracting osteotomy (PSO) and bilateral SIJ fusions.
Clinical course
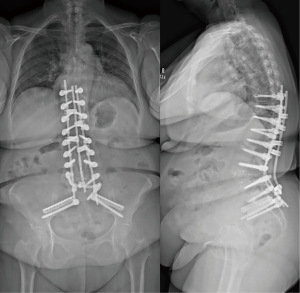
A standard midline posterior approach to the lumbar spine was utilized. Exposure of the lumbar spine was obtained in standard fashion from T10 to the sacrum. Intra-operative navigation was utilized. Pedicle screws were placed bilaterally from T10 to S1 (excluding L4) along with bilateral S2 Alar-Iliac (AI) screws. SIJ fusion was then performed aided by stealth navigation. A navigated awl was used to create a tract from the sacrum across the SIJ into the ilium proximal to the S2 AI screws. Next a guide pin was placed into this tract and over-drilled to decorticate the joint. Lastly, the iFuse-TORQ implant (Medtronic, Dublin, Ireland), packed with demineralized bone matrix (DBM), was placed into final position. Moments after insertion of the implant, the anesthesiologist notified the surgical team that the patients oxygen saturation levels dropped, blood pressure fell, and end-tidal carbon-dioxide (CO2) had decreased. This was quickly resolved. The contralateral SIJ was fused using the same technique. Again, immediately after placement of the device, the patient’s oxygen levels, blood pressure decreased, end-tidal CO2 diminished, and became mildly tachycardic. The anesthesiologist gave an intravenous fluid bolus and started low dose vasopressors. The patient’s hemodynamic status quickly returned to baseline. After discussion with the anesthesia team, we felt this was most consistent with fat emboli syndrome, but given the quick return to hemodynamic baseline, felt that surgery could proceed. The rest of the procedure was completed without issue, including completion of the three-column PSO (Figure 2). The patient was woken from anesthesia and admitted to the orthopedic floor. Her post-operative course was unremarkable, and she was discharged on post-operative day 3 without the need for oxygen. The patient had no further pulmonary complications during the course of their recovery (Figure 3).
Case 2
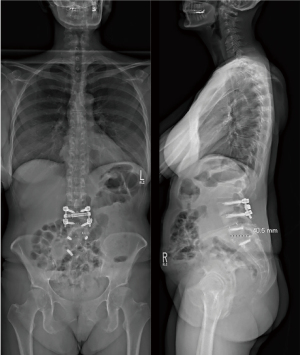
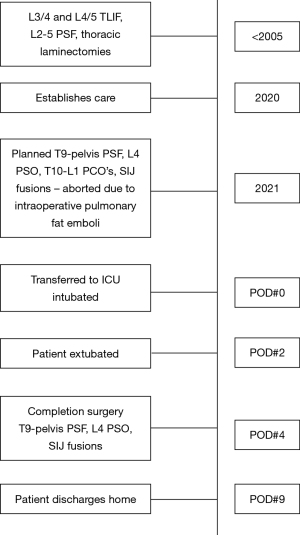
The patient was a 71-year-old female with a past medical history of osteoporosis, congestive heart failure, dilated cardiomyopathy, rheumatoid arthritis on chronic steroids, and hypertension. The patient presented to our clinic with three years of increasing back pain, bilateral lower extremity (right worse than left) radiculopathy, and symptoms consistent with lumbar spinal stenosis. Many years prior the patient underwent L2–L5 PSF with L3–L4 and L4–L5 transforaminal lumbar interbody fusions (TLIF), as well as multiple thoracic laminectomies. Preoperative imaging demonstrated a solid fusion from L2–L5, adjacent level disease with multi-level disc degeneration, spondylosis, disc protrusions, lumbar lordosis of 54 degrees, pelvic incidence of 85 degrees, and sagittal vertical axis of 12 cm (Figure 4). After discussion with the patient, decision was made to proceed with removal of implants, T9-pelvis PSF with L4 PSO and T10–L1 posterior column (Smith-Peterson) osteotomies (PCOs) to correct her deformity.
Clinical course
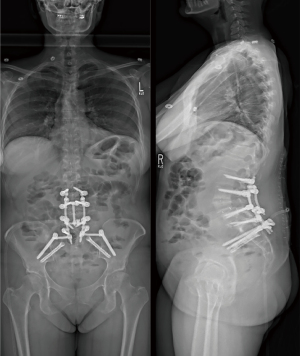
A standard midline posterior approach to the lumbar spine was utilized. Exposure of the spine was obtained in standard fashion from T9 to S2 and the posterior ilium bilaterally. Utilizing image-based navigation, pedicle screws were placed bilaterally from T10 to L3 and L5 to S1. SIJ fusion was then performed simultaneously and bilaterally utilizing navigation. A navigated awl was used to create a tract from the sacrum across the SIJ into the ilium. Next a guided pin was placed into this tract and over-drilled to decorticate the joint. A broach was used to further decorticate the joint and establish the corridor for the implant. Lastly, the iFuse triangular titanium implant (Medtronic, Dublin, IR), packed with DBM, was placed into final position. This was completed bilaterally. Moments after insertion of the second implant, the anesthesiologist notified the surgical team that the patients oxygen saturation levels dropped, blood pressure fell, and both tidal volumes and end-tidal CO2 decreased. Intraoperative blood loss to this point had only been 300 mL. Pulses were no longer palpable, and cardiopulmonary resuscitation was begun. Posterior chest compressions were started, the anesthesiologist started vasopressors, and hyperventilated the patient with 100% oxygen. A few “stay” sutures were placed in the lumbar fascia, and Io-ban was placed over the surgical wound. The patient was transferred to a hospital bed and anterior chest compressions started. Return of spontaneous cardiac activity and palpable pulses were achieved within 2 minutes. An intraoperative transesophageal echocardiogram was performed demonstrating right heart strain, consistent with a pulmonary embolus. The patient was turned on their side, the Io-ban was replaced with a wound-vac system, and the patient was transferred to the surgical intensive care unit (SICU). A post-operative chest computed tomography (CT)-angiography demonstrated no observable pulmonary embolus, providing further evidence of a transient fat embolus. The patient was started on broad spectrum antibiotics given concern for aspiration pneumonia. Laboratory studies did not demonstrate evidence of a myocardial infarction. Given the constellation of events and without signs of pulmonary thromboembolism on CT imaging, the team felt the only explanation for the code event was transient fat embolism syndrome. The patient’s vital signs quickly improved in the SICU and was extubated on post-operative day 2. On post-operative day 4, the patient returned to the operating room for completion of her surgery, which was uneventful. She successfully underwent T9-ilium PSF with L4 pedicle subtraction osteotomy and T10–L1 Smith-Peterson osteotomies (Figure 5). Post-operatively the patient was immediately extubated after completion of the procedure. On the floor, the patient quickly weaned from oxygen, worked with physical and occupational therapy, and was discharged home on hospital day 10. The patient had no further pulmonary complications during the course of their recovery (Figure 6).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from both patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Fat embolism syndrome is a potentially lethal, albeit rare, complication of both orthopedic injuries and surgeries. Classically, fat embolism syndrome is seen in the setting of long-bone fractures, especially bilateral injuries (15). There are also reports of fat embolism syndrome associated with cement extravasation, although this needs to be differentiated from a similar but different syndrome associated with polymethyl methacrylate (PMMA) (12-14).
In the spine literature, very few reports exist of fat embolism syndrome occurring during spinal instrumentation (7-11). Two other reports comment on fatal pulmonary fat embolism syndrome that occurred in the acute post-operative period (16,17). To our knowledge, there are no reports of fat embolism syndrome previously reported with implantation of a titanium SIJ fusion device, or specifically the iFuse triangular implant or iFuse-TORQ fixation device. Two separate randomized-controlled clinical trials did not report a single incidence of fat emboli in their combined cohort of over 150 patients (18,19). Our report marks the first two known reports of such an occurrence in the literature. Fortunately, both patients described in this report survived, although one of the patients did require premature termination of her index surgery, cardiopulmonary resuscitation with chest compressions, a second surgery to complete the spinal fusion and osteotomies, and an extended hospital stay.
Classically, fat embolism syndrome presents with the triad of respiratory distress, neurologic changes, and a petechial rash (15). Intraoperatively however, establishing the correct diagnosis can be much more difficult. Electrocardiogram evidence of a pulmonary embolism, whether fat emboli or thrombus, include signs of right ventricular strain, ST and/or T wave changes, and atrial abnormalities (8). Respiratory signs of fat embolism syndrome include hypoxemia, decreased lung compliance, and decreased end-tidal CO2 levels (8,15,20). Cardiovascular abnormalities include hypotension, tachycardia, and possibly non-perfusing cardiac rhythms such as ventricular fibrillation, ventricular tachycardia, or pulseless electrical activity, and complete loss of pulses (8,15,20). Intraoperative transthoracic or transesophageal echocardiography can demonstrate signs of right heart failure, while the latter can demonstrate an identifiable thrombus within the main pulmonary vasculature (8,20). Intraoperative angiography is usually not a viable option. Fat embolism syndrome is reported to be fatal in 5–20% of cases (15).
Treatment of fat embolism syndrome is largely supportive, as there is no specific medication that can be given to reverse the pathology or to remove the fat emboli from the pulmonary vasculature (8,15). To maintain oxygen saturations, the patient often must be hyperventilated with 100% oxygen. Vasopressor support is mandatory to sustain the patient’s blood pressure to ensure continued perfusion of both the spinal cord and the brain (15). In the event of loss of pulses or a non-perfusing cardiac rhythm, chest compressions and standard advance life support protocols should be initiated immediately. While these measures are largely dictated by the anesthesiology team, it is important for the spine surgeon to be well-versed with the various measures that should be taken. Probably the most useful action the surgeon can take is to ensure the spinal column is stable, rapidly pack and cover the wound with a sterile dressing to minimize bleeding and protect the surgical site, and assist in transferring the patient to a hospital bed where continued life-saving measures are performed much more easily. Thrombolytic therapy should be considered if there is concern for thrombotic rather than fat emboli as the source of the pulmonary embolus. Albumin can be given as it binds to free fatty acids (21).
Unfortunately, the reason for why fat emboli syndrome occurred in these specific patients is not entirely clear. However, given the immediate temporal relation between implantation of the devices and hemodynamic collapse in both patients, we do believe that placement of the implants was likely the precipitating factor in both patients. Of course, this is impossible to prove outright. We hypothesize that the SIJ fusion device may carry a higher risk for this complication for a few reasons. The implant, either the 3D-printed triangular implant or the screw, may act similarly to intra-medullary devices in terms of marrow disturbance. In the case of the triangular implant, it is placed by impacting the implant along a guide wire into a broached corridor in the bone. As is seen with intramedullary nails, implantation of the device may displace the bone marrow and fat out of the path of the device and into circulation (22). Most importantly, the SIJ fusion implants are typically of a larger diameter than other pelvic fixation options such as iliac bolts. Any increase in the diameter of an implant placed through the sacrum will increase the volume of marrow displacement to the power of two (cylinder volume = πr2h). Thus, the larger SIJ fusion devices may inherently carry a higher risk of emboli. Additionally, in our technique, the device is placed through the sacral ala into the posterior ilium, two regions of bone that have a relatively high ratio of bone marrow (23). Placing the implant in this area may thus run the risk of displacing fat and marrow globules into the venous circulation. When performed for primary SIJ fusion, the implant is placed from the ilium into the sacrum. In our cases, the implant was placed from sacrum into the ilium as part of our spino-pelvic fixation. While this may theoretically lead to an increased volume of the sacral marrow being disturbed in comparison to a primary SIJ fusion technique, there is currently no literature quantifying sacral-volume replacement differences between the two techniques. This could be an interesting area of future research. Another consideration is given the higher density of cortical bone within the ilium, implant placement through the ilium could possibly lead to higher intramedullary pressures than placement through the sacrum, which we believe could increase risk of emboli at this location. If so, then implant placement via either direction previously discussed would carry a risk of emboli. Lastly, it is possible that DBM particulate embolized to the lungs in these cases, similar to what was seen in the report from Morimoto where necrotic bone fragments were found embolized to the lung and other organs at autopsy (9). However, this was only used in the triangular implant, not in the 3D-printed screws used in case one.
For now, this complication seems to be rare, and should likely not dissuade the spine surgeon from use of the devices in the appropriate setting. However, we suggest that a discussion between the surgical and anesthesia teams prior to implantation of the device may help prevent complications. Ensuring that the patient’s hemodynamic status is optimized may be of benefit. Additionally, for any patients undergoing spinal surgery with intraoperative permissive hypotension, a brief increase in the patient’s blood pressure prior to implantation of the device may be considered. Lastly, as is seen in total joint arthroplasty, an additional irrigation step of the implant corridor may help decrease risk of embolism (15).
Conclusions
For patients undergoing lumbosacral fusion for degenerative scoliosis or other disorders of the lumbar spine, SIJ fusion is typically indicated when pre-existing SIJ dysfunction is present. Multiple options exist for fusion techniques and devices, including 3D-printed titanium implants. We report the first two known incidences of fat embolism syndrome associated with implantation of a SIJ titanium fusion device. The spine surgeon should be aware of this rare but potentially fatal complication and may consider intraoperative measures in coordination with their anesthesia team or additional irrigation of the implant corridor to help prevent disastrous outcomes.
Acknowledgments
Special thanks to Christina Khat, BS (University of Colorado at Anschutz Medical Center) for her assistance in manuscript preparation and submission.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-23-87/rc
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-23-87/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-23-87/coif). D.C.O.Y. reports the grants from Globus, Medtronic, SeaSpine; Royalties from SeaSpine; consulting fees from Medtronic, SeaSpine. He also received the payment from Medtronic Advisory Board for Participation on a Data Safety Monitoring Board or Advisory Board. C.J.K. reports the grants from Globus, Medtronic, SeaSpine, Medacta, Personalized Spine Study Group, Synergy, SI Bone and consulting fee from Medtronic, Allosource, SeaSpine Inc., Biocomposites. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from both patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- York PJ, Kim HJ. Degenerative Scoliosis. Curr Rev Musculoskelet Med 2017;10:547-58.
- Manzetti M, Ruffilli A, Barile F, et al. Sacroiliac Joint Degeneration and Pain After Spinal Arthrodesis: A Systematic Review. Clin Spine Surg 2023;36:169-82.
- Nakashima H, Kanemura T, Satake K, et al. Sacroiliac Joint Degeneration After Lumbopelvic Fixation. Global Spine J 2022;12:1158-64.
- Anwar FN, Nie JW, Hartman TJ, et al. Presentation, diagnosis, and treatment of sacroiliac joint dysfunction. Contem Spine Surg 2023;24:1-5.
- Sarkar M, Maalouly J, Ruparel S, et al. Sacroiliac Joint Fusion: Fusion Rates and Clinical Improvement Using Minimally Invasive Approach and Intraoperative Navigation and Robotic Guidance. Asian Spine J 2022;16:882-9.
- Martin CT, Haase L, Lender PA, et al. Minimally Invasive Sacroiliac Joint Fusion: The Current Evidence. Int J Spine Surg 2020;14:20-9.
- Takahashi S, Kitagawa H, Ishii T. Intraoperative pulmonary embolism during spinal instrumentation surgery. A prospective study using transoesophageal echocardiography. J Bone Joint Surg Br 2003;85:90-4. [Crossref] [PubMed]
- Holland R, Houten JK, Elsamragy S, et al. Intraoperative Thrombolysis of Massive Pulmonary Embolus During Spine Surgery: Case Report of Survival Complicated by Massive Bleeding and Review of the Literature. World Neurosurg 2021;146:59-63. [Crossref] [PubMed]
- Morimoto T, Kobayashi T, Yoshihara T, et al. Fatal fat embolism syndrome during posterior spinal fusion surgery: A case report and literature review. Medicine (Baltimore) 2021;100:e28381. [Crossref] [PubMed]
- Urban MK, Jules-Elysee KM, Beckman JB, et al. Pulmonary injury in patients undergoing complex spine surgery. Spine J 2005;5:269-76. [Crossref] [PubMed]
- Takahashi Y, Narusawa K, Shimizu K, et al. Fatal pulmonary fat embolism after posterior spinal fusion surgery. J Orthop Sci 2006;11:217-20. [Crossref] [PubMed]
- Ignacio JMF, Ignacio KHD. Pulmonary Embolism from Cement Augmentation of the Vertebral Body. Asian Spine J 2018;12:380-7. [Crossref] [PubMed]
- Chen HL, Wong CS, Ho ST, et al. A lethal pulmonary embolism during percutaneous vertebroplasty. Anesth Analg 2002;95:1060-2. table of contents. [Crossref] [PubMed]
- Temple JD, Ludwig SC, Ross WK, et al. Catastrophic fat embolism following augmentation of pedicle screws with bone cement: a case report. J Bone Joint Surg Am 2002;84:639-42. [Crossref] [PubMed]
- Rothberg DL, Makarewich CA. Fat Embolism and Fat Embolism Syndrome. J Am Acad Orthop Surg 2019;27:e346-55. [Crossref] [PubMed]
- Brandt SE, Zeegers WS, Ceelen TL. Fatal pulmonary fat embolism after dorsal spinal fusion. Eur Spine J 1998;7:426-8. [Crossref] [PubMed]
- Stroud MH, McCarthy RE, Parham DM, et al. Fatal pulmonary fat embolism following spinal fusion surgery. Pediatr Crit Care Med 2006;7:263-6. [Crossref] [PubMed]
- Dengler J, Kools D, Pflugmacher R, et al. Randomized Trial of Sacroiliac Joint Arthrodesis Compared with Conservative Management for Chronic Low Back Pain Attributed to the Sacroiliac Joint. J Bone Joint Surg Am 2019;101:400-11. [Crossref] [PubMed]
- Polly DW, Swofford J, Whang PG, et al. Two-Year Outcomes from a Randomized Controlled Trial of Minimally Invasive Sacroiliac Joint Fusion vs. Non-Surgical Management for Sacroiliac Joint Dysfunction. Int J Spine Surg 2016;10:28. [Crossref] [PubMed]
- Porres-Aguilar M, Rivera-Lebron BN, Anaya-Ayala JE, et al. Perioperative Acute Pulmonary Embolism: A Concise Review with Emphasis on Multidisciplinary Approach. Int J Angiol 2020;29:183-8. [Crossref] [PubMed]
- Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today 2007;37:5-8. [Crossref] [PubMed]
- Giannoudis PV, Tzioupis C, Pape HC. Fat embolism: the reaming controversy. Injury 2006;37:S50-8. [Crossref] [PubMed]
- Marx RE, Tursun R. A qualitative and quantitative analysis of autologous human multipotent adult stem cells derived from three anatomic areas by marrow aspiration: tibia, anterior ilium, and posterior ilium. Int J Oral Maxillofac Implants 2013;28:e290-4. [Crossref] [PubMed]