
Paired and matched analysis of neurological outcomes in revision surgery for cervical myelopathy following delayed neurological decline
Highlight box
Key findings
• Revision surgery for cervical myelopathy due to neurological decline results in good neurological recovery upon comparison to the index surgery and a matched patient control.
What is known and what is new?
• Neurological decline may occur following successful decompression surgery for cervical myelopathy, due to causes such as adjacent/skip level degeneration as well as restenosis at the operated levels.
• This work describes for the first time paired and matched neurological outcomes in patients receiving revision decompression due to neurological decline.
What is the implication, and what should change now?
• Our results suggest a good neurological prognosis following revision decompression in patients who are relatively young, are surgically fit, and suffer from mild-to-moderate cervical myelopathy.
Introduction
Cervical myelopathy is a common cause of spinal cord dysfunction resulting from chronic mechanical compression. Upon aging, narrowing of the cervical spinal canal results from degenerative changes which include disc herniation, facet hypertrophy, and osteophyte formation (1). Younger populations particularly in Asian locales may develop ossification of posterior longitudinal ligament (OPLL) which is another cause of cervical myelopathy (2). Patients may present with neurological deficits affecting the four limbs, gait disturbance, and impaired sphincter function. Surgery remains the mainstay of treatment for those with considerable neurological impairment or rapid progression (3). Significant improvements in functional outcomes and disability scores have been demonstrated following surgical decompression (4).
Recent long-term studies have demonstrated that most patients receiving decompression for cervical myelopathy exhibit long term neurological benefits, with those experiencing sustained neurological improvement at 10-years approaching 80% (5,6). However, delayed neurological deterioration is known to occur, and recurrent myelopathy may result from adjacent or skip level disease as well as re-stenosis at the operated level. A revision rate of 1.6–6.3% has been reported following surgery for cervical myelopathy (5,7-9). However, these studies did not specifically address how patients faired after a successful first operation, as reported causes of revision included incomplete decompression at the operated level and iatrogenic cervical instability. Here, we define ‘revision surgery’ as the necessity for a second decompression procedure in patients who experienced neurological improvement post-operation, with the etiology due to restenosis not limited to the previously operated levels but also at adjacent and skip levels. With the aging population and rise in cervical surgical volume, such revision surgeries for delayed neurological decline is anticipated to increase. We hypothesized that the spinal cord would recover poorly after repeat episodes of compression since underlying pathological changes such as glial scarring, demyelination, and neuronal apoptosis may accumulate (10). Upon revision decompression, patients are older and likely suffer from an increased number of comorbidities, factors which are known to adversely affect neurological outcomes (11,12). Furthermore, technical difficulties may be encountered in revisions due to the presence of spinal implants, altered cervical anatomy, and tissue adhesions (13).
The purpose of the current study was to determine neurological outcomes after revision surgery for cervical myelopathy. Trajectories for neurological recovery were compared between revision and the primary surgery in the same patient, as well as to a control matched for age, gender, myelopathy severity, and surgical approach. We present this article in accordance with the STROBE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-23-116/rc).
Methods
Patient selection criteria
For this retrospective study, we identified all patients who underwent revisions for cervical myelopathy in our institute between 2009 and 2020. This period was selected to ensure patients had received a minimum of 2 years of post-operative follow-up, and to facilitate electronic retrieval of cases in accordance with diagnosis/surgical procedure since earlier records were not digitized. Inclusion criteria comprised of (I) a clinical and radiological diagnosis compatible with cervical myelopathy, (II) patients having undergone two operations for the above diagnosis, (III) both surgeries being performed at our academic center, and (IV) revision performed at least 2 years after the primary surgery. The last criterion was set to exclude cases where repeated decompression was performed as a salvage procedure for inadequate decompression or due to early complications arising from the first surgery. Late neurological deterioration was identified following clinical evidence of (I) worsening of residual neurological deficits after the first surgery, and/or (II) new-onset neurological symptoms (i.e., upper limb numbness, reduced hand dexterity, gait instability). Magnetic resonance imaging (MRI) was obtained in all patients prior to the primary procedure and revision to confirm radiological cervical canal stenosis and to facilitate surgical planning. Exclusion criteria included cervical cord compression from causes other than degeneration/disc prolapse/OPLL, previous operations on the cervical spine for diseases other than cervical myelopathy, absence of detailed peri-operative neurological assessment, and presence of pre-existing neurological diseases (i.e., cerebrovascular events, hereditary neuropathies, neuromuscular diseases) that affected limb and sphincter function. We also excluded cases where there was a clear traumatic cause for acute deterioration (i.e., fall resulting in central cord syndrome). With the exception of one patient who defaulted clinical attendance at 12 months, all patients had at least 24 months of follow-up after their revision surgery. This study was approved by the Institutional Review Board (IRB) of our tertiary academic hospital center (protocol UW 20-583). Informed consent was waived as anonymized retrospective data was utilized. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013).
Data collection
Clinical and radiological data was retrospectively collected from electronic records where available and were supplemented with information from case files. Collected details included age at surgery, gender, presence of OPLL, etiology of neurological decline requiring revision, and surgical details (i.e., approach, procedure, number of cervical levels decompressed).
The modified Japanese Orthopedic Association (mJOA) scale was utilized as the primary measure of peri-operative neurological recovery for both the first surgery as well as upon revision. The assessment was conducted by an experienced occupational therapist within one month of the operation. Post-operative mJOA scores were similarly obtained at 1, 3, 6, 12-months post-operation, and yearly thereafter. The peak post-operative mJOA score was denoted as the highest score achieved at up to 2 years from the date of operation since scores have been reported to plateau within 12 months after surgery (14). Due to scheduling difficulties, patients were not all seen at exactly 12 months after surgery, and therefore peak post-operative mJOAs were taken as a point of comparison for maximal recovery.
The differences between pre-operative and post-operative mJOA scores were calculated to obtain the change in mJOA score (ΔmJOA) and recovery rate (RR), and to identify whether a minimum clinically important difference (MCID) was reached. RRs were calculated as described by Hirabiyashi, using the formula (mJOA postoperative − mJOA preoperative)/(17− mJOA preoperative) ×100 (15). An MCID of 2.5 for mJOA scores following treatment has been established previously (16). This was utilized as the threshold for non-inferiority testing with pooled variance, and the difference in the pre- and post-operative mJOA scores were calculated between comparison groups to determine whether the non-inferior margin was attained (i.e., ΔmJOA after revision − ΔmJOA after primary surgery).
Matching design and statistical analysis
Revision vs. primary operation
Clinical and surgical details as well as mJOA trajectories were retrieved from the first operation and revision surgery for the 14 patients fulfilling the recruitment criteria. Differences between pre-operative and peak post-operative mJOA scores, ΔmJOA, and RR was analyzed using the Mann-Whitney U test. The proportion of patients achieving the MCID of 2.5 (16) was analyzed using Chi-square testing. Patients with missing neurological parameters were excluded from the corresponding analysis.
Revision group vs. matched group
The 14 patients were matched at the time of revision to a control group receiving a single operation at a 1:1 ratio adjusted at the time of surgery for age (±5 years), gender, presence of OPLL, pre-operative mJOA scores (±2), and surgical approach. Similarly, differences between pre-operative and peak post-operative mJOA scores, ΔmJOA and RR were analyzed using the Mann-Whitney U test. The proportion of patients achieving the MCID was analyzed using Chi-square testing.
Statistics analysis and reporting framework
IBM SPSS Statistics (version 28.0.1.0) and SAS JMP Statistical software (version 17.1) were utilized for statistical analysis. Continuous variables with normal distributions are described by mean values ± standard deviation. The threshold for statistical significance was set at P<0.05 (two-tailed testing). This case series has been reported in line with the PROCESS guideline (17). Upon encountering missing values these were excluded from analysis.
Results
Patient demographics and revision details
We identified 14 patients (13 males and 1 female) requiring revision surgery for cervical myelopathy according to the inclusion and exclusion criteria. Mean age at the time of the first operation was 54.6±11.4 years (range: 41–77 years). The posterior approach was adopted in 11 patients during the first surgery, and the anterior approach in the remaining three patients. Congenital cervical stenosis was common in our population, leading to a large proportion of patients developing multilevel stenosis and indicated to receive posterior decompression upon possessing a favorable lordotic cervical alignment. The number of cervical levels decompressed ranged from 1 to 4. Mean age at the time of revision was 61.4±11.0 years (range, 49–83 years) which was an average of 6.8±4.2 years (range, 2–14 years) after the first operation. Upon revision, the posterior approach was favored in nine patients, the anterior approach in four, whilst one patient received combined anterior and posterior decompression. Seven patients (50%) had their revisions performed via the same approach as in the primary operation. Details are compiled in Table 1.
Table 1
| Variable | Primary operation | Revision | Matched control |
|---|---|---|---|
| Age at surgery (years) | 54.6±11.4 | 61.4±11.0 | 62.9±12.2 |
| Gender | |||
| Male | 13 | 13 | 13 |
| Female | 1 | 1 | 1 |
| Symptom duration (months) | 20.2±25.8 | 17.4±14.3 | 9.5±11.6 |
| Follow-up duration (years) | 6.8±4.2# | 5.4±3.9 | 5.4±4.5 |
| Surgical approach | |||
| Anterior | 5 | 5 | 5 |
| Posterior | 9 | 9 | 9 |
| Pre-operative mJOA | 10.4±3.3 | 9.8±2.2 | 9.3±2.8 |
Variables are presented as mean ± standard deviation or number. #, duration between primary operation and revision. mJOA, modified Japanese Orthopaedic Association.
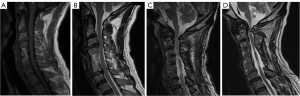
The cause for neurological decline requiring revision was attributed to adjacent level disease in 8 patients (57.1%), recurrent stenosis at the operated levels in 4 patients (28.6%) and skip level disease in 2 patients (14.3%). It was confirmed via screening of medical records that the four patients diagnosed with recurrent stenosis at the operated levels exhibited an initial improvement in symptomatology and mJOA scores after surgery but deteriorated later. In these patients, increase in disc herniation, osteophyte formation, and ‘spring back’ closure following suture laminoplasty (18) were identified as associated causes. Examples of pathology are shown in Figure 1. At the time of revision surgery, anterior corpectomy/discectomy and fusion was performed in five patients, laminoplasty was performed in two, and laminectomy and fusion performed in six (one of which required concomitant C1 arch excision). C1 arch excision alone was performed in one patient.
Neurological outcomes following revision vs. primary operation
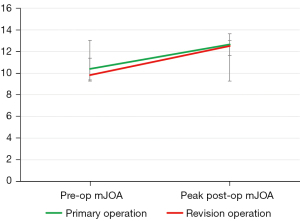
Pre-operative and peak post-operative mJOA scores for the primary operation were 10.4±3.3 and 12.6±2.9 respectively. Of 14 patients, 13 who underwent revision operations due to delayed neurological decline exhibited a second surge in mJOA score (range, 0.5–7) after re-decompression, as shown in Table 2. When comparing the magnitude of mJOA improvement of the index versus the revision surgery, Mann-Whitney U testing demonstrated that the increment in mJOA was similar between two operations with no statistical difference of note (P=0.536), i.e., an absolute increase in mJOA score of 2.7±2.0 (range, −0.5 to 6) was achieved after revision as compared to of 2.2±2.1 (range, −1 to 5.5) after the primary operation. The trend in pre-operative and peak post-operative mJOA scores is illustrated in Figure 2. For both operations, MCID was attained in approximately 50% of patients (46.1% for the index surgery and 50% for the revision surgery). The mean mJOA recovery ratios were 35.0% and 38.1% at the index and revision surgeries respectively. The mean duration for patients to reach peak post-operative mJOA scores was 7.8±2.9 months after the index operation, and 8.7±3.1 months after the revision operation (P=0.678). No complications, such as infection, hematoma formation, early post-operative neurological deterioration, or iatrogenic stability occurred following revision surgery at the time of latest follow-up.
Table 2
| Outcome | Primary operation | Revision | P value |
|---|---|---|---|
| Pre-operative mJOA | 10.4±3.3 | 9.8±2.2 | 0.107 |
| Post-operative mJOA | 12.6±2.9 | 12.5±2.1 | 0.202 |
| ΔmJOA | +2.2±2.1 | +2.7±2.0 | 0.536 |
| Recovery ratio (%) | 35.0±37.4 | 38.1±25.4 | 0.867 |
| Achievement of MCID | 6/13 (46.2)† | 7/14 (50.0) | 0.842 |
Variables are presented as mean ± standard deviation or number (percentage). †, index operation mJOA scores were not retrievable in one patient. Physical records had been discarded as the surgery was conducted decades ago. mJOA, modified Japanese Orthopaedic Association score; ΔmJOA, change in mJOA score; MCID, minimum clinically important difference.
Neurological outcomes following revision vs. matched control
The revision group was matched to control patients based on demographic, clinical and surgical factors (Table 1). Differences in age at the time of operation in patients receiving surgery compared to matched patients receiving primary decompression were insignificant (61.4±11.0 vs. 62.9±12.2, P=0.723), as were pre-operative mJOA scores (9.8±2.2 vs. 9.3±2.8, P=0.635). Follow-up duration was similarly robust at 5.4±3.9 (range, 1–13) years from the revision surgery and 5.4±4.5 (range, 1–16) in the matched controls (P=0.990). Symptom duration prior to surgery was shorter in the matched group (9.5±11.6 months) as compared to the revision group (17.4±14.3 months) but this was not statistically different (P=0.176).
Upon comparison of mJOA scores for the revision surgery and a matched control (Table 3), we observed that the revision cohort attained a mean peak post-operative mJOA that was comparable to the control group, at 12.5±2.1 vs. 12.5±3.1 (P=0.946). The revision group exhibited an absolute increase in mJOA score of 2.7±2.0 (range, 0.0–5.0), while the matched group had an increase of 3.2±2.8 (range, −1.5–9.0). The difference in improvement was not statistically significant (P=0.571). RR was 38.1%±25.4% in the revision group and 39.3%±32.6% in the matched group (P=0.910). Seven patients in the revision group attained an MCID of 2.5 (50%) within 2 years compared to eight patients in the matched group (57.1%), which was similarly statistically insignificant (P=0.705). The time to reach peak mJOA was 9±2.4 months for the matched group compared to 8.7±3.1 months after revision surgery (P=0.500).
Table 3
| Outcome | Revision | Matched control | P value |
|---|---|---|---|
| Pre-operative mJOA | 9.8±2.2 | 9.3±2.8 | 0.635 |
| Post-operative mJOA | 12.5±2.1 | 12.5±3.1 | 0.946 |
| ΔmJOA | +2.7±2.0 | +3.2±2.8 | 0.571 |
| Recovery ratio (%) | 38.1±25.4 | 39.3±32.6 | 0.910 |
| Achievement of MCID | 7/14 (50.0) | 8/14 (57.1) | 0.705 |
Variables are presented as mean ± standard deviation or number (percentage). mJOA, modified Japanese Orthopaedic Association; ΔmJOA, change in mJOA score; MCID, minimum clinically important difference.
Non-inferiority testing
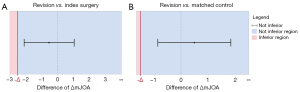
The difference in change of mJOA score after surgery was used for non-inferiority testing. Comparing change in mJOA following revision with the same patient’s primary surgery achieved a mean difference of −0.536 (95% CI: −2.103 to 1.031) which was in the non-inferior region (Figure 3A) with the MCID set at 2.5 (P=0.02). Comparing mJOA increase following revision to a matched patient receiving primary surgery achieved a mean difference of 0.486 (95% CI: −0.863 to 1.835) which was again located in the non-inferior region (Figure 3B) with the expected margin of increase in mJOA after surgery set at 1.5 (P<0.02). When set at the MCID of 2.5, non-inferiority was demonstrated at P<0.01 upon comparison of revision and matched subjects.
Discussion
The volume of surgery for cervical myelopathy has been exponentially rising (19,20) and long-term studies demonstrate that most patients have lasting neurological and functional benefits (5,6). Nevertheless, revisions for late neurological decline remain inevitable in a proportion of patients, and such surgeries are also expected to increase in number (5). To the best of our knowledge, our present study contains the first comprehensive cohort to-date whereby neurological outcomes have been assessed longitudinally following both a primary decompression surgery for cervical myelopathy as well as a revision surgery upon late neurological deterioration.
Our results demonstrated that patients were able to ‘get back on track’ after a revision surgery and make up for the lost gains in neurological function. Neurological improvement upon revision was demonstrated in our cohort to average a mJOA increase of 2.7, whilst MCID was attained in 50% of patients. The increase in mJOA score experienced upon revision even exceeded that of the primary surgery (2.7 vs. 2.2), and the capacity to reach an MCID of 2.5 demonstrated non-inferiority. Our mJOA gains experienced from both operations compare favorably with related literature. Naderi et al. reported a mJOA increase of 2.2 (12.2 vs. 14.4) over a mean follow-up period of 54 months (21), whilst Cheung et al. (4) reported a mJOA increase of 2.4 (10.0 vs. 12.4) with plateau in scores attained between 6–12 months post-operation. Assessment of neurological function at 10 years post-operation suggest that the extent of neurological improvement diminishes over time, with an average mJOA increase of just 1.1 compared to pre-operative assessment (14.2 vs. 13.1) (6). Predictors for neurological survivorship following decompression surgery for cervical myelopathy include age at operation (12), duration of symptoms (22), neurological severity (23), medical comorbidities, and smoking (11). Findings from our cohort are especially applicable towards supporting revision surgery for younger and fitter patients (average age of 61.4 years) suffering from moderate myelopathy (average mJOA score of 9.8), but may be of less relevance to the middle-old onwards (i.e., 75 years and over) with severe neurological deficits.
Epidemiological aspects of revision following surgery for cervical myelopathy have been well-described. Complications are not uncommon upon revision, although none were observed in our modest cohort of 14 patients. Amongst 623 patients receiving laminoplasty and followed-up for 6 years, 10 (1.6%) required reoperation beyond 6 months after the initial surgery (8). Six reoperations were performed for C5 palsy and radiculopathy. Restenosis due to instability after laminoplasty occurred in one case, and enlargement of OPLL in three cases. Another cohort of 222 patients receiving cervical laminoplasty revealed a 6.3% revision rate outside of the acute postoperative period with an average follow-up period of 5 years, in which only 1.3% of reoperations were due to recurrent myelopathy (24). Following anterior cervical decompression and fusion, a population-based study identified a cumulative revision rate of 2.5% at 4 years, although the cause for revision was unspecified (7). Subgroup analysis demonstrated a higher risk of reoperation for patients receiving anterior surgery for myelopathy as compared to radiculopathy, and other risk factors included male gender, diabetes, and treatment at tertiary hospitals (7). Gok et al. described neurological outcomes using Nurick scores alone in 30 patients with cervical spondylotic myelopathy undergoing revision surgery for multiple indications including pseudoarthrosis, instability, hardware failure and recurrent stenosis. Post-operative Nurick scores demonstrated improvement in 25/30 over 19 months, but a complication rate of 27% (13). Liu described the results of secondary laminoplasty after failed anterior surgery for cervical myelopathy, of which 8/17 patients were suffering from progressive disease (25). At 6 months, average mJOA scores had increased by 3.6. For these last two studies, no comparison was made to the primary surgery.
Limitations
Our current study had several limitations. First, the size of the case series was small, which affected statistical power and did not allow for the identification of prognostic factors following revision surgery. Secondly, there was selection bias in patients receiving reoperation since individuals who had an acceptable level of functional despite neurological decline or were surgically unfit due to comorbidities would be counselled towards conservative management. Finally, as quality of life measures were not regularly documented, these were absent from this retrospective study. A strength in being a single center study however was relative homogeneity with regards to surgical indications and planning, as well as peri-operative neurological assessment which was conducted by the same team of doctors and therapists.
Conclusions
This is the first study to evaluate the neurological outcomes of revisions for recurrent cervical myelopathy with reference to both the index surgery as well as a matched operation. Revisions were found to offer substantial neurological improvement, returning patients to near peak mJOA levels prior to post-operative decline, whilst not causing surgical complications of note. Considering the global increase of decompressive surgery for cervical myelopathy, cases of late neurological deterioration indicated for revision are expected to rise. Whilst these findings need to be consolidated upon larger sample sizes, they facilitate decision making and counselling during clinical practice.
Acknowledgments
Funding: This study was funded by charitable donations received from the ‘Get up and walk’ campaign awarded to G.K.H.S. and P.A.K.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-23-116/rc
Data Sharing Statement: Available at https://jss.amegroups.com/article/view/10.21037/jss-23-116/dss
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-23-116/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-23-116/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of our tertiary academic hospital center (protocol UW 20-583). Informed consent was waived as anonymized retrospective data was utilized. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nouri A, Tetreault L, Singh A, et al. Degenerative Cervical Myelopathy: Epidemiology, Genetics, and Pathogenesis. Spine (Phila Pa 1976) 2015;40:E675-93. [Crossref] [PubMed]
- Inamasu J, Guiot BH, Sachs DC. Ossification of the posterior longitudinal ligament: an update on its biology, epidemiology, and natural history. Neurosurgery 2006;58:1027-39; discussion 1027-39. [Crossref] [PubMed]
- Edwards CC 2nd, Riew KD, Anderson PA, et al. Cervical myelopathy. current diagnostic and treatment strategies. Spine J 2003;3:68-81. [Crossref] [PubMed]
- Cheung WY, Arvinte D, Wong YW, et al. Neurological recovery after surgical decompression in patients with cervical spondylotic myelopathy - a prospective study. Int Orthop 2008;32:273-8. [Crossref] [PubMed]
- Yick VHT, Zhang C, Wong JSH, et al. Neurological Survivorship Following Surgery for Degenerative Cervical Myelopathy: A Longitudinal Study on 195 Patients. J Bone Joint Surg Am 2023;105:181-90. [Crossref] [PubMed]
- Dijkman MD, van Bilsen MWT, Fehlings MG, et al. Long-term functional outcome of surgical treatment for degenerative cervical myelopathy. J Neurosurg Spine 2022;36:830-40. [Crossref] [PubMed]
- Park MS, Ju YS, Moon SH, et al. Reoperation Rates After Anterior Cervical Discectomy and Fusion for Cervical Spondylotic Radiculopathy and Myelopathy: A National Population-based Study. Spine (Phila Pa 1976) 2016;41:1593-9. [Crossref] [PubMed]
- Nakashima H, Kanemura T, Satake K, et al. Reoperation for Late Neurological Deterioration After Laminoplasty in Individuals With Degenerative Cervical Myelopathy: Comparison of Cases of Cervical Spondylosis and Ossification of the Posterior Longitudinal Ligament. Spine (Phila Pa 1976) 2020;45:E909-16. [Crossref] [PubMed]
- Fontaine-Pérus J, Halgand P, Chéraud Y, et al. Mouse-chick chimera: a developmental model of murine neurogenic cells. Development 1997;124:3025-36.
- Ogino H, Tada K, Okada K, et al. Canal diameter, anteroposterior compression ratio, and spondylotic myelopathy of the cervical spine. Spine (Phila Pa 1976) 1983;8:1-15. [Crossref] [PubMed]
- Kim HJ, Moon SH, Kim HS, et al. Diabetes and smoking as prognostic factors after cervical laminoplasty. J Bone Joint Surg Br 2008;90:1468-72. [Crossref] [PubMed]
- Nakashima H, Tetreault LA, Nagoshi N, et al. Does age affect surgical outcomes in patients with degenerative cervical myelopathy? Results from the prospective multicenter AOSpine International study on 479 patients. J Neurol Neurosurg Psychiatry 2016;87:734-40. [Crossref] [PubMed]
- Gok B, Sciubba DM, McLoughlin GS, et al. Revision surgery for cervical spondylotic myelopathy: surgical results and outcome. Neurosurgery 2008;63:292-8; discussion 298. [Crossref] [PubMed]
- Suzuki A, Misawa H, Simogata M, et al. Recovery process following cervical laminoplasty in patients with cervical compression myelopathy: prospective cohort study. Spine (Phila Pa 1976) 2009;34:2874-9. [Crossref] [PubMed]
- Hirabayashi K, Miyakawa J, Satomi K, et al. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine (Phila Pa 1976) 1981;6:354-64. [Crossref] [PubMed]
- Kato S, Oshima Y, Matsubayashi Y, et al. Minimum Clinically Important Difference and Patient Acceptable Symptom State of Japanese Orthopaedic Association Score in Degenerative Cervical Myelopathy Patients. Spine (Phila Pa 1976) 2019;44:691-7. [Crossref] [PubMed]
- Agha RA, Sohrabi C, Mathew G, et al. The PROCESS 2020 Guideline: Updating Consensus Preferred Reporting Of CasESeries in Surgery (PROCESS) Guidelines. Int J Surg 2020;84:231-5. [Crossref] [PubMed]
- Wang HQ, Mak KC, Samartzis D, et al. "Spring-back" closure associated with open-door cervical laminoplasty. Spine J 2011;11:832-8. [Crossref] [PubMed]
- Marquez-Lara A, Nandyala SV, Fineberg SJ, et al. Current trends in demographics, practice, and in-hospital outcomes in cervical spine surgery: a national database analysis between 2002 and 2011. Spine (Phila Pa 1976) 2014;39:476-81. [Crossref] [PubMed]
- Kotkansalo A, Leinonen V, Korajoki M, et al. Surgery for degenerative cervical spine disease in Finland, 1999-2015. Acta Neurochir (Wien) 2019;161:2147-59. [Crossref] [PubMed]
- Naderi S, Ozgen S, Pamir MN, et al. Cervical spondylotic myelopathy: surgical results and factors affecting prognosis. Neurosurgery 1998;43:43-9; discussion 49-50. [Crossref] [PubMed]
- Tetreault L, Wilson JR, Kotter MRN, et al. Is Preoperative Duration of Symptoms a Significant Predictor of Functional Outcomes in Patients Undergoing Surgery for the Treatment of Degenerative Cervical Myelopathy? Neurosurgery 2019;85:642-7. [Crossref] [PubMed]
- Naruse T, Yanase M, Takahashi H, et al. Prediction of clinical results of laminoplasty for cervical myelopathy focusing on spinal cord motion in intraoperative ultrasonography and postoperative magnetic resonance imaging. Spine (Phila Pa 1976) 2009;34:2634-41. [Crossref] [PubMed]
- Rodriguez-Feo JA, Leas D, Odum SM, et al. Reoperation Rates Following Open-Door Cervical Laminoplasty. Int J Spine Surg 2018;12:751-6. [Crossref] [PubMed]
- Liu HW, Chen L, Xu NW, et al. Outcomes of secondary laminoplasty for patients with unsatisfactory results after anterior multilevel cervical surgery. J Korean Neurosurg Soc 2015;57:36-41. [Crossref] [PubMed]


