
Spinal epidural hematoma and permanent paraplegia following spinal cord stimulator implantation: a case report
Highlight box
Key findings
• Rare case report of spinal cord stimulators (SCS) placement leading to spinal epidural hematoma with resulting permanent paraplegia.
• Even with decompression within 24 hours, the reversal of paraplegia may not be guaranteed.
What is known and what is new?
• Reoperation after SCS is common, often due to lead migration or misplacement; SCS have significant risks such as epidural hematoma, but permanent deficits are rarely reported. The benefits of SCS beyond placebo are unclear, with recent Cochrane reviews questioning previous literature, and a recent randomized control trial showing no difference between SCS and placebo.
• Swift identification and debridement, while crucial, may not always guarantee the resolution of severe complications, such as paraplegia. Traditional pre-operative coagulation assessments may prove insufficient in evaluating the bleeding risk among individuals with a history of liver transplant, potentially imparting a misleading perception of hemostatic stability.
What is the implication, and what should change now?
• Restrict SCS use to controlled trials pending additional research on therapeutic benefits.
• Conduct short-term and long-term studies on SCS patients, particularly those with complex medical histories, to assess complications and long-term risks. Implement closer outpatient follow-up within 3 days of SCS placement to identify potential complications early.
Introduction
Spinal cord stimulators (SCS) are increasingly recognized as a successful therapy for chronic pain, providing an alternative to long-term reliance on opioid medications. This sizable patient demographic presents significant opportunities for advancements in the field, benefiting both patients and corporations offering these alternative solutions. The use of SCS has seen substantial growth in the past two decades. The global market for spinal cord stimulation devices is projected to reach US $2.8 billion by 2025 (1).
The exploration of targeting the spinal cord to modulate pain was initially introduced in a groundbreaking paper published by Science in 1965 (2). Over time, various treatments have emerged, including injection-based therapies and radiofrequency ablation, aiming at the spinal cord. While both treatment modalities may provide short-lived benefits, they often require frequent and ongoing procedures. In contrast, spinal cord stimulation aims to overcome these limitations by offering longer-term pain relief with fewer necessary interventions.
The placement of a SCS entails a subcutaneous implantable pulse generator connected to two electrodes, with leads extending into the epidural space posterior to the spinal cord dorsal columns. Although the exact mechanism underlying the pain-alleviating effects of SCS remains not fully understood, animal studies indicate the significance of the inhibitory neurotransmitter γ-amino butyric acid (GABA) in SCS analgesia (3).
As the popularity of SCS rises, the literature has raised questions about therapeutic benefits, leading to an increased focus on complications. The rates of complications are still quite debatable, but multiple large retrospective studies have consistently identified hardware-related issues as the most common postoperative complication. A recent study by Papadopoulos et al. highlighted lead migration or misplacement as the most frequent indication for reoperation (4). While serious adverse events are rare, they significantly impact SCS usage. A study by West et al. specifically examined hematoma incidence, revealing 0.81% overall and 0.32% neuraxial (5).
Several innovations in SCS focus on reducing complication rates. A widely accepted risk mitigation method involves a trial period for patient selection, where electrodes are temporarily placed in the epidural space, keeping the generator outside the body. In other innovative areas, the settings and electrical properties like high frequency and burst SCS have been hypothesized to have an impact on complications (6). Additionally, dorsal root ganglion (DRG) stimulation has shown a decreased incidence of lead migration (7).
This case report is crucial as it underscores a severe complication linked to SCS. Recent systematic reviews have raised doubts about previous conclusions, stressing the necessity for additional studies with strong methodologies. The severe adverse event highlighted in this report should serve as a cautionary signal regarding the use of SCS. Considering the current literature and this case report, it might be advisable to limit the use of SCS to trials until a definitive therapeutic benefit is established.
The objective of this report is to scrutinize existing literature to discern the therapeutic efficacy, or lack thereof, of SCS. Emphasis will be placed on the documented occurrences of permanent deficits associated with SCS in the literature, and strategies to mitigate these potential risks in the future. We present this article in accordance with the CARE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-23-139/rc).
Case presentation
A 70-year-old married Caucasian male presented to the emergency room in distress, experiencing acute bilateral leg weakness, sensory deficits, and difficulty urinating 3 days after receiving a permanent SCS. His medical history included type 2 diabetes, hypertension, and a history of liver adenocarcinoma, with a liver transplant in February 2023. He had a complex surgical history, including a diagnostic abdominal laparoscopy in 2019 and lumbar spine surgery in 1980. The patient, a former alcohol abuser, ceased alcohol intake after being diagnosed with liver cirrhosis in 2022. Chronic back pain was his main concern post-liver transplant.
Five months before undergoing SCS placement, the patient sought the spine surgeon for the management of chronic back pain. The surgeon diagnosed the patient with thoracic and lumbar spondylosis, radiculopathy, costal chondritis, and intercostal neuralgia. Traditional pain medications were limited due to contraindications post-liver transplant. Six weeks of conservative therapy and two epidural spinal injections showed minimal improvement. A trial of the SCS resulted in a self-reported 70% pain reduction at an appointment 1 week following the placement. The decision for permanent surgery was made, performed without complications on September 25th, with same-day discharge.
Upon arrival at the emergency room 2 days following the permanent placement of the SCS, the patient underwent a comprehensive evaluation, including a neurological assessment and radiological studies. The neurological examination performed by the emergency room (ER) physician revealed bilateral 1/5 strength in the lower extremities, with significantly diminished reflexes bilaterally. Bilateral radicular paresthesia was observed below the T9 nerve distribution. Inspection of the surgical site showed it to be clean, with no notable warmth or erythema. Minimal tenderness to palpation was noted at the surgical site. The computed tomography (CT) scan indicated significant edema surrounding the previous surgical site; however, the presence of hardware made it challenging to form a clear clinical impression. These evaluations paired with his recent history of placement of a SCS revealed an alarming scenario: the possibility of an acute spinal epidural hematoma.
The orthopedic surgeon who had placed the SCS was consulted. Due to the critical nature of the situation the patient was taken to the operating room for immediate surgical exploration and intervention 3 days after the permanent placement of the SCS. Intraoperative findings revealed a hematoma at the laminectomy bed, which was promptly evacuated. The SCS device was carefully removed, and exploration of the battery pack site uncovered another sizable hematoma. All wounds were methodically explored to address potential bleeders, and once hemostasis was achieved, the surgical wounds were closed in layers.
The emergent debridement surgery proceeded without complications, and the patient was admitted to the surgical unit for continued care. Over the next 24 hours, the orthopedic and medical services conducted frequent neurologic assessments. Unfortunately, there was no improvement in the patient’s neurologic deficits, and he continued to exhibit bilateral leg weakness and sensory deficits, as well as bladder and bowel dysfunction.
Given the persistent neurologic deficits and the high suspicion of recurrent hematoma, a magnetic resonance imaging (MRI) was obtained on the second day following the initial surgery. The MRI confirmed the presence of a large fluid collection extending from the level of T6 to the site of the laminectomy at T8, further suggesting a hematoma as the culprit for spinal cord compression. A second debridement procedure was scheduled promptly, taking place less than 36 hours after the patient’s initial presentation to the ER.
The second surgical intervention involved re-entering the previous laminectomy site at T8. The incision was extended 3 cm cephalad to the level of T6. Intraoperatively, a large hematoma was identified, compressing the spinal cord within the laminectomy bed. The hematoma was gently removed and dissected, revealing that it extended from T9 to T6. After thorough debridement, the spinal cord was palpated through the dura confirming the absence of any residual hematoma and ensuring adequate decompression. To reduce the likelihood of recurrent hematoma formation, a Jackson-Pratt drain was placed to facilitate continuous drainage of the space where the hematoma had initially formed.
Despite extensive surgical interventions illustrated in Figure 1, the patient’s neurologic deficits persisted postoperatively. The absence of sensory and motor recovery led to the recommendation of an intensive inpatient spinal cord rehabilitation program by physical therapist (PT) and occupational therapist (OT) specialists, aiming to regain leg function.
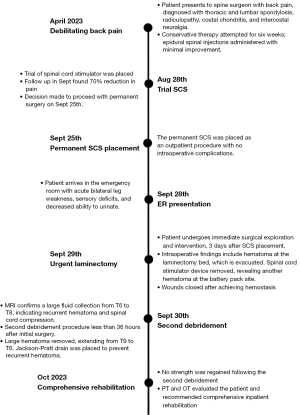
The patient’s dedication to rehabilitation was admirable, but the outlook for functional recovery is uncertain. Profound neurologic deficits and substantial spinal cord compression have had a significant impact. The situation is further complicated by a delayed diagnosis, occurring 3 days after the initial procedure, and the need for two debridement procedures. The first debridement, performed urgently, took place on the morning of the 4th day post-operatively due to the patient’s late presentation and the extensive emergency department workup.
Patient perspective
The following survey was obtained 2 months after his discharge from hospitalization.
“I’m hanging in there. Though my leg strength hasn’t fully returned, I’m adjusting to my new reality, which comes with added challenges. Who would have thought I would be able to get a liver transplant with less deficits than this spine stimulator. Despite the difficulties, I’ve been grateful for the amazing care and support from my medical team and loved ones throughout this journey.”
Ethical statement
To ensure full compliance with ethical standards and respect for the patient’s autonomy, a meticulous procedure was followed. Initially, the patient was approached, and the purpose, scope, and potential benefits of the case report, along with any associated risks or implications, were explained. Addressing any questions or concerns the patient may have had. Upon the patient expressing willingness to be included, a detailed written informed consent form was presented. This document delineated the study’s objectives, the patient’s rights, and the confidentiality of personal information. The patient had the opportunity to review the form, seek clarification, and make an informed decision without any undue pressure. Following the acquisition of their signature, the consent form was securely stored to maintain patient confidentiality and adhere to ethical guidelines. This procedural approach ensured the patient’s autonomy and rights were respected throughout the development of the case report. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images.A copy of the written consent is available for review by the editorial office of this journal.
Discussion
While the theoretical framework behind these devices appears highly promising, the data supporting their efficacy is less conclusive. The literature on SCS presents a distinct dichotomy, possibly attributed to the robust theoretical foundation coupled with compelling incentives for the companies involved.
The growing utilization of spinal cord stimulation can be linked to misleading assertions propagated by studies marked with significant bias and flawed methodologies (1). In a 2019 study by Lamer et al., spinal stimulation showed a higher responder rate and pain improvement, but 11 of the 12 studies were industry-sponsored. The only non-industry sponsored study found no difference in outcomes. The overall quality assessment using AMSTAR-2 rated it as moderate (8). Another study in 2020 by Duarte et al. reported a reduction in pain scoring with a greater effect than placebo. However, this review received a low to moderate AMSTAR-2 rating due to conflicts of interest among authors and poor methodology (9).
In 2021, O’Connell et al. published an intervention review with a robust methodology. In their study, they found all randomized control trial (RCT) results to have a high risk of bias (10). They also found poor quality and large inconsistencies in the reporting of adverse events. The authors’ conclusions were: “We found very low-certainty evidence that SCS may not provide clinically important benefits on pain intensity compared to placebo stimulation.” (10).
Following the insightful findings of O’Connell et al., a clear demand arose for unbiased randomized control trials. In response, Hara et al. conducted a placebo-controlled, crossover, randomized clinical trial published in JAMA in 2022. Involving 50 patients, the study revealed no significant difference in self-reported back pain change from baseline (11). Notably, Hara et al.’s study was the first to publish intermediate results of such a trial. At the 6-month follow-up, no significant difference in pain-related disability was observed among the 34 patients who completed post-trial follow-up (12).
Long-term outcomes were also assessed in the large retrospective comparative analysis by Dhruva et al. (13). In the propensity-matched population of 7,560 patients, they found SCS placement was not associated with a reduction in opioid use or nonpharmacologic pain interventions at 2 years.
Expanding upon O’Connell et al.’s seminal systematic review, Traeger et al. conducted a comparable review. Like O’Connell’s study, Traeger identified a significant prevalence of high detection and performance bias in the randomized control trials they examined, attributed to insufficient blinding and selective reporting (14). The authors drew similar conclusions from their findings: “Data in this review do not support the use of SCS to manage low back pain outside a clinical trial. Current evidence suggests SCS probably does not have sustained clinical benefits that would outweigh the costs and risks of this surgical intervention.” (14).
While extensively promoted as non-invasive and generally safe in various literature papers, SCS carry the potential for causing severe disability. Notably, there is a limited amount of reporting in the literature on permanent paraplegia arising from a SCS. To our knowledge, occurrences of hematoma in relation to SCS have been infrequently mentioned, and permanent paraplegia has never been discussed in the literature. Furthermore, a more thorough evaluation of the literature reveals that studies without financial interests show minimal therapeutic benefits.
This case report highlights the serious consequences of spinal cord epidural hematomas, an extremely uncommon event. Remarkably, the deficits observed in this case did not show swift resolution after decompression, which is a rarity in itself. Another notable aspect of this report is the detailed exploration of the patient’s medical history and the discussion surrounding their eligibility as a SCS candidate.
The literature on hematomas associated with spinal cord stimulation is limited. A retrospective analysis of 12,297 patients reported only 15 cases of hematomas within a 90-day period (15). A systematic review by West et al. found a hematoma incidence of less than 1%, with neuraxial hematomas occurring in less than 0.5% of cases (5). Notably, the study did not report any instances of permanent paraplegia due to neuraxial hematomas; a single case reported paraplegia, but the patient recovered after debridement with no significant deficits (16).
In the absence of specific guidelines for spinal epidural hematomas post SCS placement, we can adopt the recommended approach for managing spontaneous spinal epidural hematomas to mitigate potential adverse outcomes. A multicenter study conducted by Fukui et al. underscored the significance of the time elapsed from onset to debridement as a critical prognostic factor. In their findings, surgery was typically conducted around 24 hours after the onset of symptoms (17).
The American Society of Pain and Neuroscience (ASPN) acknowledges the need for clinical guidance in patients with chronic bleeding disorders. Lee et al. highlight the inadequacy of relying solely on common coagulation tests for pre-surgical assessment (18). The ASPN recommends detailed discussions and patient education, particularly in cases involving chronic bleeding disorders.
The indications for the SCS trial were vague, with the patient managing chronic back pain with opiates and experiencing improvement with steroid injections. Minimal precautions were taken for severe medical comorbidities. The main precaution implemented involved the trial period of SCS. However, evaluating the effectiveness of the SCS trial encountered limitations due to its exceptionally short-term follow-up, potentially influenced by the placebo effect.
Our case emphasizes the importance of impartially recognizing and assessing the risks associated with medical comorbidities when evaluating therapeutic benefits. In addition to preoperative discussions, it underscores the significance of promptly addressing neurological deficits following SCS placement to prevent severe consequences resulting from delayed diagnosis and treatment. To mitigate the risk of spinal epidural hematoma, particularly in patients with bleeding disorders, we concur with Traeger et al. in advocating for the restriction of SCS use to clinical trials until a proven therapeutic benefit is established (14).
Conclusions
This case underscores the imperative for exercising heightened caution when considering the utilization of SCS. The gravity of the serious and enduring side effects necessitates thorough deliberation when opting for SCS over medical management in the treatment of chronic back pain. Our case report emphasizes the need for surgeons to reassess the widespread application of SCS, advocating for its reserved use in controlled trials until the therapeutic benefits are unequivocally established.
While SCS have the potential to offer pain relief, it is imperative not to underestimate the potential complications, such as spinal epidural hematoma. There is a call for further research to gain a deeper understanding of the genuine therapeutic benefits and to comprehensively evaluate the potential for serious complications in both the short and long term.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-23-139/rc
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-23-139/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-23-139/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Knotkova H, Hamani C, Sivanesan E, et al. Neuromodulation for chronic pain. Lancet 2021;397:2111-24. [Crossref] [PubMed]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150:971-9. [Crossref] [PubMed]
- Guan Y, Wacnik PW, Yang F, et al. Spinal cord stimulation-induced analgesia: electrical stimulation of dorsal column and dorsal roots attenuates dorsal horn neuronal excitability in neuropathic rats. Anesthesiology 2010;113:1392-405. [Crossref] [PubMed]
- Papadopoulos DV, Suk MS, Andreychik D, et al. Rates and Causes of Reoperations Following Spinal Cord Stimulation Within a 2-12 year Period. Global Spine J 2023; Epub ahead of print. [Crossref] [PubMed]
- West T, Driver CN, D'Souza RS. Incidence of Neuraxial and Non-Neuraxial Hematoma Complications From Spinal Cord Stimulator Surgery: Systematic Review and Proportional Meta-Analysis. Neuromodulation 2023;26:1328-38. [Crossref] [PubMed]
- Braun E, Khatri N, Kim B, et al. A Prospective, Randomized Single-Blind Crossover Study Comparing High-Frequency 10,000 Hz and Burst Spinal Cord Stimulation. Neuromodulation 2023;26:1023-9. [Crossref] [PubMed]
- Esposito MF, Malayil R, Hanes M, et al. Unique Characteristics of the Dorsal Root Ganglion as a Target for Neuromodulation. Pain Med 2019;20:S23-30. [Crossref] [PubMed]
- Lamer TJ, Moeschler SM, Gazelka HM, et al. Spinal Stimulation for the Treatment of Intractable Spine and Limb Pain: A Systematic Review of RCTs and Meta-Analysis. Mayo Clin Proc 2019;94:1475-87. [Crossref] [PubMed]
- Duarte RV, Nevitt S, McNicol E, et al. Systematic review and meta-analysis of placebo/sham controlled randomised trials of spinal cord stimulation for neuropathic pain. Pain 2020;161:24-35. [Crossref] [PubMed]
- O'Connell NE, Ferraro MC, Gibson W, et al. Implanted spinal neuromodulation interventions for chronic pain in adults. Cochrane Database Syst Rev 2021;12:CD013756. [PubMed]
- Hara S, Andresen H, Solheim O, et al. Effect of Spinal Cord Burst Stimulation vs Placebo Stimulation on Disability in Patients With Chronic Radicular Pain After Lumbar Spine Surgery: A Randomized Clinical Trial. JAMA 2022;328:1506-14. [Crossref] [PubMed]
- Hara S, Andresen H, Solheim O, et al. Six-Month Follow-up of a Trial of Spinal Cord Burst Stimulation vs Placebo Stimulation and Disability in Patients With Chronic Radicular Pain After Lumbar Spine Surgery. JAMA 2023;329:1985-6. [Crossref] [PubMed]
- Dhruva SS, Murillo J, Ameli O, et al. Long-term Outcomes in Use of Opioids, Nonpharmacologic Pain Interventions, and Total Costs of Spinal Cord Stimulators Compared With Conventional Medical Therapy for Chronic Pain. JAMA Neurol 2023;80:18-29. [Crossref] [PubMed]
- Traeger AC, Gilbert SE, Harris IA, et al. Spinal cord stimulation for low back pain. Cochrane Database Syst Rev 2023;3:CD014789. [PubMed]
- Labaran L, Jain N, Puvanesarajah V, et al. A Retrospective Database Review of the Indications, Complications, and Incidence of Subsequent Spine Surgery in 12,297 Spinal Cord Stimulator Patients. Neuromodulation 2020;23:634-8. [Crossref] [PubMed]
- Fan X, Ren H, Lu Z. Epidural hematoma with dramatical recovery: A rare severe complication of spinal cord stimulation. Asian J Surg 2022;45:2460-1. [Crossref] [PubMed]
- Fukui H, Kamei N, Fujiwara Y, et al. Prognostic factors for spontaneous spinal epidural hematoma: a multicenter case-control study. Acta Neurochir (Wien) 2022;164:1493-9. [Crossref] [PubMed]
- Lee JM, Lee D, Christiansen S, et al. Spinal Cord Stimulation in Special Populations: Best Practices from the American Society of Pain and Neuroscience to Improve Safety and Efficacy. J Pain Res 2022;15:3263-73. [Crossref] [PubMed]


