
Single position lateral lumbar interbody fusion with navigated percutaneous pedicle screw fixation: technique modification with resultant resource usage optimisation
Highlight box
Key findings
• Single position (SP) anterior-to-psoas (ATP) lateral lumbar interbody fusion (LLIF) is associated with a decreased length of stay, shorter operative time and lower consumables fee.
What is known and what is new?
• SP ATP LLIF has been demonstrated to be a safe alternative to the conventional dual position (DP) ATP LLIF.
• Resource usage comparison has not been made between SP and DP ATP LLIF. To our knowledge, this is the first study comparing cost between the two techniques.
What is the implication and what should change now?
• A technique modification was able to result in reduced resource usage. More in-depth studies should be conducted to evaluate long-term outcomes of SP compared to DP ATP LLIF.
Introduction
Over the years, healthcare spending has exhibited a consistent upward trend on a global scale (1,2). This complex issue is influenced by various determinants encompassing government policies, the structure of healthcare financing, and the escalating costs associated with medical equipment and healthcare personnel (3,4). These multifaceted factors often lie beyond the realm of most healthcare practitioners. However, clinicians possess a distinctive vantage point from which they can actively contribute to the containment and efficient management of healthcare expenditures through their clinical practices (5).
In surgical practice, the operating theatre (OT) is a resource intensive environment, with studies reporting OT costs to be the second most expensive contributor to a patient’s surgical care (6). Recent evidence has suggested that variations in surgeon’s experience and preference influenced OT resource usage (7). In the field of orthopaedics, studies on resource optimisation have mostly focused on joint replacement surgeries (8-10). The literature on cost analysis of spine surgeries is sparse.
Recent data indicate a notable surge in the volume of elective lumbar fusion surgeries. Concurrently, there has also been a noticeable upwards trend in the average cost associated with these procedures (11). Among the various techniques for lumbar fusion, lateral lumbar interbody fusion (LLIF) emerges as a highly effective minimally invasive surgical choice for patients afflicted with degenerative lumbar disease (12,13). LLIF is routinely performed with posterior instrumentation for improved biomechanical stability of the segment (14). Traditionally, LLIF and posterior instrumentation were staged procedures. However, technique advancements have enabled some surgeons to perform both interventions in a single operative session, albeit necessitating two different patient positions, lateral decubitus for LLIF and a prone position for percutaneous pedicle screw (PPS) insertion (15). The most recent evolution in this surgical technique has resulted in both LLIF and posterior instrumentation being executed in a singular patient position (16-19).
With the elimination of positional changes during this single-staged, single-position LLIF procedure, the authors postulate that there will be a reduction in resource utilization. The principal objective of this study was to assess the aforementioned hypothesis. We present this article in accordance with the STROBE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-24-20/rc).
Methods
This was a retrospective cohort study conducted in a tertiary academic hospital in Singapore with ethics approval obtained from the Singhealth Centralised Institutional Review Board (IRB), approval number 2023/2505. Informed consent was waived as de-identified data was used for the analysis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
All patients who underwent single-stage anterior-to-psoas (ATP) LLIF by the senior author between September 2020 and September 2023 were identified. Patients who underwent revision fusion surgery were excluded. Demographic details included were gender, age and Charlson Comorbidity Index (CCI). Operative variables collected were duration of surgery and if surgery was performed in a single position (SP) or dual position (DP). Complications evaluated were intra-operative complications such as dura tear, injury to nerve roots and anaesthesia related complications. Peri-operative complications included were bleeding requiring blood transfusion, deep vein thrombosis or pulmonary embolism, myocardial infarction, pneumonia, urinary tract infection, delirium, unplanned return to theatre, superficial surgical site infection and inpatient mortality. Variables related to resource usage that were of interest included length of stay (days), implant fee (Singapore dollars, SGD), consumables fee (SGD), anaesthetist fee (SGD) and facility fee (SGD).
Statistical analysis
Stata 14.0 (College Station, TX, USA) was used for statistical analysis. The distribution characteristics of each group were explored, including skewness, mean, and type of distribution. To compare quantitative outcomes between groups, the Mann-Whitney U-test was utilised, presenting results as medians with interquartile range (IQR). As the outcome measures were skewed, they were log-transformed to approximately conform to normality.
To provide a comprehensive view beyond median comparison, quantile regression was employed, assessing differences across various quantiles of the data. Additionally, Generalized Linear Models (GLMs) with suitable distribution patterns (e.g., Gamma) were utilized to account for the nature of our outcome variables. These models were adjusted for CCI and levels. Significance level was defined as P<0.05.
Operative procedure
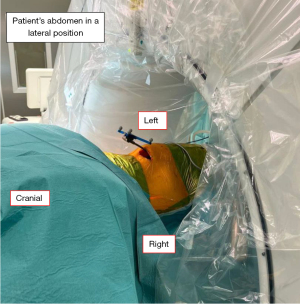
After intubation, the patient was placed in a right lateral decubitus position with hips in slight flexion to relax the psoas muscle. The target segment was identified using intra-operative c-arm fluoroscopy. Following which, the surgical field was cleaned and draped. This included the planned location for pedicle screw fixation (Figure 1). After skin incision, the external oblique, internal oblique and transversus abdominis muscles were bluntly dissected for access to the retroperitoneal space. The psoas muscle was identified and retracted posteriorly. With the extraperitoneal fat acting as a cushion, the abdominal organs were retracted anteriorly. Once the intervertebral space was visualised, a guide needle was inserted and position was confirmed with fluoroscopy. A tubular retractor with an attached light source was then inserted into the surgical plane to facilitate access. This was followed by annulotomy and disc space preparation. A combination of curette, end-plate shaver, pituitary rongeur and Kerrison rongeur were used to ensure thorough disc space preparation. Following which, trial implants were inserted. Once the desired implant size was selected, the actual implant with allograft was inserted. Fluoroscopy was used to check the position of implant and once it was satisfactory, the surgical site was irrigated, Floseal used for haemostasis, and closure was performed in layers.
SP
When performing SP LLIF, the patient remains in the right lateral decubitus position. The reference array was then inserted on the left iliac crest. Following which, the O-arm® (Medtronic plc.; Surgical Technologies, Louisville, USA) was brought into the surgical field and draped, before being brought to the vertebral levels of interest (Figure 2). Anteroposterior and lateral radiographs were performed to confirm the level followed by a computer tomography scan. The data was then transferred to the navigation system (StealthStation S8; Medtronic, Minneapolis, MN, USA). A planar probe was used to guide the skin incisions for the insertion of the pedicle screws. After skin incision, a navigated Jamshidi needle (CareFusion, San Diego, CA, USA) was used to create a pedicle tract. A Kirschner wire was passed through the Jamshidi needle to maintain the trajectory. Following which the Jamshidi needle was removed. The cannulated pedicle screw was inserted via the Kirschner wire using a navigated screw driver (Figure 3). The wire was removed once the screw was in place. Rods were inserted and radiographs were performed using the O-arm®. Once position was satisfactory, the rods were locked in place. A final set of radiographs was performed prior to closure.
DP
In DP LLIF, after closure of the abdominal incision, all drapes were removed. The patient was then turned prone. Following which, all members of the surgical team performed surgical scrubbing and gowning again. The surgical field for posterior instrumentation was then clean and draped. Surgical exposure was performed for attachment of the reference array on the spinous process in greatest proximity to the vertebral levels of interest. The next steps for O-arm® positioning and navigated screws insertion were similar to what was described above.
Post-operative instructions
All patients were reviewed by the physiotherapist on the same day or on post-operative day one. They were allowed ambulation with lumbar corset post-operatively. Radiographs were performed after patients ambulated. They were discharged after completion of three doses of IV antibiotics and when cleared by physiotherapist for home.
Results
A total of 20 patients were identified. There were 6 patients (5 males, 1 female) in the SP group and 14 patients (9 males, 5 females) in the DP group. The median age of patients in the SP group was 67 [62, 72] years while the median age of patients in the DP group was 66 [58, 71] years. There were no significant difference in the distribution of the CCI between the two groups (P=0.27). Among the six patients in the SP group, 4 (66.6%) had single level fusion, 1 (16.7%) had two level fusion and another patient (16.7%) had three level fusion. Among the 14 patients In the DP group, 5 (35.7%) had single level fusion, 6 (42.9%) had two level fusion and 3 (21.4%) had three level fusion. There was no significant difference in the distribution of levels of fusion between the two groups (P=0.57). None of the patients had intra-operative complications. There were no recorded post-operative complications in the SP group, but one patient in the DP group developed pneumonia post-operatively. The above is summarised in Table 1.
Table 1
| Variable | All | Single position | Dual position | P value |
|---|---|---|---|---|
| No. of patients | 20 | 6 (30.0) | 14 (70.0) | – |
| Age (years) | 66 [62, 71.5] | 67 [62, 72] | 66 [58, 71] | 0.71 |
| Gender | 0.61 | |||
| Male | 14 (70.0) | 5 (83.3) | 9 (64.3) | |
| Female | 6 (30.0) | 1 (16.7) | 5 (35.7) | |
| CCI | 0.27 | |||
| 0 | 9 (45.0) | 1 (16.7) | 8 (57.1) | |
| 1 | 5 (25.0) | 2 (33.3) | 3 (21.4) | |
| 2 | 6 (30.0) | 3 (50.0) | 3 (21.4) | |
| Levels | 0.57 | |||
| 1 | 9 (45.0) | 4 (66.6) | 5 (35.7) | |
| 2 | 7 (35.0) | 1 (16.7) | 6 (42.9) | |
| 3 | 4 (20.0) | 1 (16.7) | 3 (21.4) | |
| Patients with post-operative complications | 1 (5.0) | 0 (0.0) | 1 (7.1)—pneumonia | >0.99 |
Data are presented as median [IQR] or n (%). CCI, Charlson Comorbidity Index; IQR, interquartile range.
The median length of stay for both the SP and DP groups was four days. However, from the GLM model, the SP group was associated with a 44.6% decrease in the length of stay (P=0.02) compared to the DP group, holding CCI and levels constant. The median operative time for the SP group was 150 min (IQR, 140–240 min) while the operative time for the DP group was 282.5 min (IQR, 210–420 min), P<0.001. The median implant fee ($15,815 vs. $20,371, P=0.91), consumables fee ($2,509 vs. $3,839, P<0.001) and anaesthetist fee ($188 vs. $216, P=0.43) for the SP group were all lower than the DP group although only the difference in consumables fee achieved statistical significance. When comparing the median facility fee, the SP group was slightly higher at $1,553 compared to $1,467, but this was not statistically significant (P=0.85). When comparing the median sum of implants, consumables, anaesthetist and facility fees between the two groups, SP had a lower cost ($21,881, IQR, 19,290–30,902) compared to DP ($32,297, IQR, 23,031–35,640), P=0.24. More details can be found in Table 2.
Table 2
| Variable | Single position (n=6) | Dual position (n=14) | Adjusted coefficient | 95% CI | P value |
|---|---|---|---|---|---|
| Length of stay (days)* | 4 [2, 4] | 4 [4, 12] | −0.59 | −1.09, −0.08 | 0.02 |
| Operative time (minutes)* | 150 [140, 240] | 282.5 [210, 420] | −0.10 | −1.0, −0.24 | <0.001 |
| Implant fee (SGD)* | 15,815 [14,700, 21,900] | 20,371 [15,020, 25,820] | −0.001 | −0.02, 0.02 | 0.91 |
| Consumables fee (SGD)* | 2,509 [2,091, 2,940] | 3,839 [3,032, 4,479] | −0.05 | −0.08, −0.03 | <0.001 |
| Anaesthetist fee (SGD) | 188 [103, 305] | 216 [215, 616] | −0.05 | −0.16, 0.07 | 0.43 |
| Facility fee (SGD)* | 1,553 [866, 2,391] | 1,467 [1,426, 4,358] | −0.007 | −0.08, 0.07 | 0.85 |
| Sum of implant, consumables, anaesthetist and facility fees (SGD)* | 21,881 [19,290, 30,902] | 32,297 [23,031, 35,640] | −0.01 | −0.03, 0.01 | 0.24 |
Data are presented as median [IQR]. *, adjusted for levels and CCI. SGD, Singapore dollars; CI, confidence interval; IQR, interquartile range; CCI, Charlson Comorbidity Index.
Discussion
The rising healthcare cost is not unique to the spine service, but also reflected in the greater healthcare system (1,2). As the aging population continue to drive healthcare expenses, there is a growing imperative to seek innovative solutions for cost containment. In this paper, we have demonstrated a reduction in operative time as well as cost in single level LLIF when performed in a SP compared to DP.
LLIF has been gaining popularity as a minimally invasive technique for treating degenerative lumbar spinal conditions. It allows for the implantation of a larger fusion cage with increased fusion rate (20), while avoiding posterior structures in the spine such as the nerve roots and ligamentum flavum. This technique also facilitates indirect decompression and is able to restore both sagittal and coronal alignment (21). Figure 4 demonstrates pre-operative lumbar spine radiographs of a patient who underwent SP LLIF by the senior author, while Figure 5 demonstrates the post-operative lumbar spine radiographs of the same patient.
When performed with the conventional technique, the patient is required to be in two positions, lateral for LLIF and prone for posterior instrumentation. The surgery may be staged, or done in a single setting with positional change intra-operatively. With further developments in this surgical technique, spine surgeons were able to perform the procedure with the patient remaining in a SP throughout the surgery (16-19). It was reported by Ziino et al. that DP LLIF had a longer OT time compared to SP and was likely due to the intra-operative position change (18). This result is consistent with what we have demonstrated in this study. The reduction in OT time has also resulted in a reduction of anaesthetist fee. Repositioning intra-operatively can also be associated with complications from anaesthesia such as endotracheal tube displacement (22). None of the DP patients in this study had such complications due to the meticulous care provided by the anaesthetists. With the elimination of position changes in SP LLIF, the usage of consumables was also reduced. Consumables include a new set of sterile drapes, new set of surgical gown and gloves for all staff scrubbing up for the case, as well as prone face cushions and ocular paddings for the patient. All these add up to a significant cost for patients and the healthcare service. From the results in this study, the total cost of implants, consumables, anaesthetist fee and facility fees was more than $10,000 lower in the SP group compared to the DP group, with the most significant cost reduction achieved among consumables. The SP group was also associated with a shorter length of acute hospital stay compared to the DP group after correcting for CCI and number of levels fused. This outcome may be attributed to the reduced operative time and as a result, reduced time under general anaesthesia (GA). Although GA remains the predominant anaesthesia modality in spine surgery, prolonged time under GA is associated with its own set of complications as well as increased LOS (23). In order to mitigate the effect GA has on post-operative recovery, recent advancements in spine surgery include awake spinal fusion. Previous studies have reported that awake spinal fusion was associated with a shorter time to ambulation and decreased LOS (24,25). While most studies have reported on awake transforaminal lumbar interbody fusion (TLIF), the literature on awake LLIF is sparse. Further research is warranted to evaluate awake LLIF.
While innovation for cost savings is a worthy pursuit, it must not compromise clinical outcomes. Ouchida et al. performed a cohort study comparing the outcomes of SP to DP LLIF. Like our study, PPS was inserted under navigation guidance. They reported a reduction in intra-operative time without significant difference in rates of PPS misplacement and endplate injury. The Japanese Orthopaedic Association (JOA) score was also evaluated at 6 months and there was no significant difference between the two groups (17). Another study comparing SP to DP LLIF was performed by Cheng et al., PPS insertion was performed without navigation guidance. There was no significant difference in rates of PPS misplacement. The post-operative visual analogue scale (VAS) score and Oswestry Disability Index (ODI) was also similar between the two groups (16). However in Ziino et al.’s study comparing SP to DP LLIF where PPS insertion was performed without navigation guidance, endplate fracture occurred in 7.1% of patients in the SP group and screw removal was required for 4.8% of patients in the SP group. The DP group did not have these complications (18). Although computed tomography (CT) scans were not performed post-operatively for patients in our study, none of the patients required screw removal and all post-operative radiographs were reviewed by the senior author and screw positions were satisfactory. In another study by Blizzard et al., fusion rate among patients who underwent SP LLIF was evaluated with CT at 6 months and was reported as 87.5% (19). This was similar to a meta-analysis reporting LLIF rates to be 91.6% (20).
It is worth noting that PPS placement with the patient in a lateral position is not conventional and may pose as a steep learning curve for some spine surgeons (26). If performed under fluoroscopy guidance, the rate of screw misplacement may be higher in the hands of less experienced surgeons. However, with the aid of navigation, PPS insertion in the lateral position can be performed more safely and accurately (27). From the senior author’s experience, extra care has to be taken when inserting the left pedicle screw as the effect of gravity on the soft tissues can affect the direction of the screwdriver and hence the screw position.
This study possesses several notable strengths. It comprises a consecutive series conducted by a single surgeon within a single tertiary hospital, and with a standardised post-operative recovery protocol. This eliminates potential variations that could compromise the validity of the results, enhancing the reliability and comparability of the outcomes. The potential confounding factors and the variable accuracy of data collection via a registry method were not present. The small number of patients in this study is a limitation the authors recognise. This was also a retrospective cohort study and potential factors that can influence the outcomes of interest were not fully within control.
Conclusions
SP LLIF with navigated PPS insertion described in this paper is a MIS technique with reduced resource usage. Previous studies on SP LLIF reported equivalent clinical outcomes when compared to conventional LLIF performed in two positions. In our institution, the overall cost of SP LLIF with navigated PPS was lower than single stage DP LLIF. However, more studies are required to evaluate the long term outcomes of SP LLIF.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-24-20/rc
Data Sharing Statement: Available at https://jss.amegroups.com/article/view/10.21037/jss-24-20/dss
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-24-20/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-24-20/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval was obtained from the Singhealth Centralised Institutional Review Board (IRB), approval number 2023/2505. Informed consent was waived as de-identified data was used for the analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bodenheimer T. High and rising health care costs. Part 1: seeking an explanation. Ann Intern Med 2005;142:847-54. [Crossref] [PubMed]
- Matchar DB, Lai WX, Kumar A, et al. A Causal View of the Role and Potential Limitations of Capitation in Promoting Whole Health System Performance. Int J Environ Res Public Health 2023;20:4581. [Crossref] [PubMed]
- Kumar RK. Technology and healthcare costs. Ann Pediatr Cardiol 2011;4:84-6. [Crossref] [PubMed]
- Stadhouders N, Kruse F, Tanke M, et al. Effective healthcare cost-containment policies: A systematic review. Health Policy 2019;123:71-9. [Crossref] [PubMed]
- Warsame R, Riordan L, Jenkins S, et al. Responsibilities, Strategies, and Practice Factors in Clinical Cost Conversations: a US Physician Survey. J Gen Intern Med 2020;35:1971-8. [Crossref] [PubMed]
- Stey AM, Brook RH, Needleman J, et al. Hospital costs by cost center of inpatient hospitalization for medicare patients undergoing major abdominal surgery. J Am Coll Surg 2015;220:207-17.e11. [Crossref] [PubMed]
- Adkins HH, Hardacker TJ, Ceppa EP. Examining variation in cost based on surgeon choices for elective laparoscopic cholecystectomy. Surg Endosc 2016;30:2679-84. [Crossref] [PubMed]
- Hsu AR, Gross CE, Bhatia S, et al. Template-directed instrumentation in total knee arthroplasty: cost savings analysis. Orthopedics 2012;35:e1596-600. [Crossref] [PubMed]
- Mattei L, Pellegrino P, Calò M, et al. Patient specific instrumentation in total knee arthroplasty: a state of the art. Ann Transl Med 2016;4:126. [Crossref] [PubMed]
- Siegel GW, Patel NN, Milshteyn MA, et al. Cost Analysis and Surgical Site Infection Rates in Total Knee Arthroplasty Comparing Traditional vs. Single-Use Instrumentation. J Arthroplasty 2015;30:2271-4. [Crossref] [PubMed]
- Martin BI, Mirza SK, Spina N, et al. Trends in Lumbar Fusion Procedure Rates and Associated Hospital Costs for Degenerative Spinal Diseases in the United States, 2004 to 2015. Spine (Phila Pa 1976) 2019;44:369-76. [Crossref] [PubMed]
- Tung KK, Tseng WC, Wu YC, et al. Comparison of radiographic and clinical outcomes between ALIF, OLIF, and TLIF over 2-year follow-up: a comparative study. J Orthop Surg Res 2023;18:158. [Crossref] [PubMed]
- Zhu L, Wang JW, Zhang L, et al. Outcomes of Oblique Lateral Interbody Fusion for Adult Spinal Deformity: A Systematic Review and Meta-Analysis. Global Spine J 2022;12:142-54. [Crossref] [PubMed]
- Huang S, Min S, Wang S, et al. Biomechanical effects of an oblique lumbar interbody fusion combined with posterior augmentation: a finite element analysis. BMC Musculoskelet Disord 2022;23:611. [Crossref] [PubMed]
- He W, He D, Sun Y, et al. Standalone oblique lateral interbody fusion vs. combined with percutaneous pedicle screw in spondylolisthesis. BMC Musculoskelet Disord 2020;21:184. [Crossref] [PubMed]
- Cheng P, Zhang XB, Zhao QM, et al. Efficacy of Single-Position Oblique Lateral Interbody Fusion Combined With Percutaneous Pedicle Screw Fixation in Treating Degenerative Lumbar Spondylolisthesis: A Cohort Study. Front Neurol 2022;13:856022. [Crossref] [PubMed]
- Ouchida J, Kanemura T, Satake K, et al. Simultaneous single-position lateral interbody fusion and percutaneous pedicle screw fixation using O-arm-based navigation reduces the occupancy time of the operating room. Eur Spine J 2020;29:1277-86. [Crossref] [PubMed]
- Ziino C, Konopka JA, Ajiboye RM, et al. Single position versus lateral-then-prone positioning for lateral interbody fusion and pedicle screw fixation. J Spine Surg 2018;4:717-24. [Crossref] [PubMed]
- Blizzard DJ, Thomas JA. MIS Single-position Lateral and Oblique Lateral Lumbar Interbody Fusion and Bilateral Pedicle Screw Fixation: Feasibility and Perioperative Results. Spine (Phila Pa 1976) 2018;43:440-6. [Crossref] [PubMed]
- Li HM, Zhang RJ, Shen CL. Differences in radiographic and clinical outcomes of oblique lateral interbody fusion and lateral lumbar interbody fusion for degenerative lumbar disease: a meta-analysis. BMC Musculoskelet Disord 2019;20:582. [Crossref] [PubMed]
- Sato J, Ohtori S, Orita S, et al. Radiographic evaluation of indirect decompression of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lateral interbody fusion for degenerated lumbar spondylolisthesis. Eur Spine J 2017;26:671-8. [Crossref] [PubMed]
- Minonishi T, Kinoshita H, Hirayama M, et al. The supine-to-prone position change induces modification of endotracheal tube cuff pressure accompanied by tube displacement. J Clin Anesth 2013;25:28-31. [Crossref] [PubMed]
- Phan K, Kim JS, Kim JH, et al. Anesthesia Duration as an Independent Risk Factor for Early Postoperative Complications in Adults Undergoing Elective ACDF. Global Spine J 2017;7:727-34. [Crossref] [PubMed]
- Sykes DAW, Tabarestani TQ, Chaudhry NS, et al. Awake Spinal Fusion Is Associated with Reduced Length of Stay, Opioid Use, and Time to Ambulation Compared to General Anesthesia: A Matched Cohort Study. World Neurosurg 2023;176:e91-e100. [Crossref] [PubMed]
- Garg B, Ahuja K, Mehta N, et al. Awake Spinal Fusion. JBJS Rev 2021; [Crossref] [PubMed]
- Warren SI, Wadhwa H, Koltsov JCB, et al. One surgeon's learning curve with single position lateral lumbar interbody fusion: perioperative outcomes and complications. J Spine Surg 2021;7:162-9. [Crossref] [PubMed]
- Shree Kumar D, Ampar N, Wee Lim L. Accuracy and reliability of spinal navigation: An analysis of over 1000 pedicle screws. J Orthop 2020;18:197-203. [Crossref] [PubMed]






