
Clinical and radiographic outcomes after index anterior cervical discectomy and fusion with interbody spacer with integrated anchor fixation: a single-surgeon case study
Highlight box
Key findings
• Patients who underwent anterior cervical discectomy and fusion (ACDF) with plate-less, stand-alone interbody spacers with integrated anchor fixation (ISa) showed similar clinical outcomes to other integrated spacers.
What is known and what is new?
• Traditional plate-cage ACDF systems are associated with significant approach-related complications such as dysphagia.
• When compared to published literature, the ISa system used in this study demonstrates lower rates of dysphagia and readmissions than plate-cage systems and similar outcomes to other plate-less spacers.
What is the implication, and what should change now?
• ACDF with ISa cages has some approach-related benefits and is a viable alternative to traditional cage plate systems.
• The results from this study should be followed up with head-to-head controlled trials against other ACDF spacer systems.
Introduction
Anterior cervical discectomy and fusion (ACDF) is a commonly performed procedure for cervical myelopathy, radiculopathy, and spinal cord injury (1). The surgery involves dissecting the neck anteriorly, removing the intervertebral disc, decompressing the spinal cord and nerve roots, and inserting a graft to restore normal disc height and cervical lordosis (1). A typical ACDF fusion construct includes an anterior plate to hold the graft in place, but recent advances have led to the implantation of stand-alone spacers that are directly anchored or screwed into the vertebral bodies (2,3).
While ACDF procedures are generally safe, there are some common complications that can arise, the most common of which is dysphagia, or difficulty swallowing (4). The cause of postoperative dysphagia is multifactorial but has been attributed to injury to the pharyngeal innervation, adhesions, postoperative swelling, and vocal cord paralysis (5). The use of steroids to decrease postoperative swelling and dysphagia is controversial, with some studies finding that steroids reduce dysphagia while others note no change (6-8). Importantly, the use of anterior plating has been associated with increased rates of dysphagia, potentially due to the increased tissue dissection needed to place a plate and the presence of space-occupying material behind the esophagus (4,5). Stand-alone spacers without plating have also shown lower rates of postoperative dysphagia compared to plated systems (2-4).
In this case series, we characterize outcomes for patients who underwent ACDF with a titanium stand-alone interbody spacer with integrated anchor fixation (ISa) (COALITION MIS®, Globus Medical Inc., Audubon, PA, USA). We aim to describe the clinical outcomes, complications including dysphagia and reoperation, and radiographic outcomes such as global and segmental lordosis, subsidence, and fusion. We present this article in accordance with the STROBE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-24-32/rc).
Methods
Patient selection and data collection
This study was conducted as a retrospective, single cohort case series. The cohort for this study consisted of patients from one surgeon (M.M.A.) who underwent first-time ACDF surgery between January 1, 2018 and December 31, 2021 at a tertiary medical center. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board (IRB) of Duke University Medical Center under Pro00090408. Due to the retrospective nature of this study, informed consent was deemed not necessary.
Inclusion criteria were: elective surgery, patients 18 years or older, patients with at least 90 days of postoperative follow-up, and patients who underwent index ACDF using a stand-alone titanium spacer with anchoring (COALITION MIS®). Illustrations and real-world images of this spacer are shown in Figure 1. Exclusion criteria were: emergency/trauma surgery, patients who had undergone previous ACDF surgery, patients with spacers that included screws instead of anchors, and patients with anterior cervical plating.

We collected patient data via retrospective review of the electronic medical record. Baseline patient demographics were collected, including age at surgery, sex, body mass index (BMI) at the time of surgery, comorbidities (hypertension, hyperlipidemia, diabetes mellitus, heart disease, and smoking prior to surgery). Surgical data, including specific levels of surgery, surgical duration, length of hospital stay, use of intravenous intraoperative steroids, and indication for surgery were collected.
One of the collected clinical outcomes was the incidence of postoperative dysphagia. Dysphagia was defined as any report in the postoperative notes of difficulty or pain with swallowing that was changed from the patient’s preoperative baseline. To evaluate this at preoperative and postoperative visits, patients were asked about their normal ability to swallow, any new pain or difficulty with swallowing liquids and solids, or any new issues with food or liquid getting stuck in the throat. We collected this outcome immediately postoperatively, as well as at 6 weeks, 3 months, and 6 months after surgery. Other outcomes included intraoperative complications, postoperative complications, 90-day postoperative emergency department (ED) visits, 90-day postoperative hospital readmissions, and need for reoperation. We also calculated the yearly rate of adjacent segment disease (ASD) requiring reoperation by dividing the number of patients reoperated for ASD by the number of patients with eligible lengths of follow-up (9).
Radiographic data collected were global cervical lordosis (from C2–7), segmental cervical lordosis (surgical segments), anterior and posterior disc height at each operative segment, and radiographic fusion of operative segments. Radiographic fusion was defined as the presence of bridging bone between endplates and the absence of radiolucency between the interbody spacer and endplates (10). Radiographic outcomes were collected from lateral upright X-rays at preoperative baseline and the following postoperative timepoints as available: immediate, 6 weeks, 3 months, 6 months, 9 months, 1 year, and 2 years.
Statistical analysis
Most patient demographics, such as sex, surgical levels, rates of comorbidities, and surgical indications were summarized with frequencies and percentages, while age, hospital length of stay, and postoperative follow-up were reported as means with standard deviation. The proportion of patients who experienced dysphagia, complications, ED visits, and readmissions were reported with frequencies and percentages.
For each timepoint, the global and segmental lordosis in degrees was reported with mean and standard deviation, while the change in lordosis in degrees was reported up to three months postoperatively for patients who had repeated imaging. The change in disc height was reported with both absolute and percentage change from prior to surgery to post-surgery, as well as from post-surgery to 6 weeks and 3 months after for patients with repeated imaging. Studies have defined clinically significant subsidence anywhere from 2–4 mm, thus we defined surgical segments with a greater than 3 mm decrease in anterior or posterior disc height as having significant subsidence (11-15). The frequencies of radiographic fusion of all operative segments in each patient was reported for all available timepoints.
Welch’s t-test was used to compare average global and segmental lordosis of the cervical spine between baseline, 6 weeks postoperatively, and 3 months postoperatively. Due to the exploratory and retrospective nature of this study, we did not impute or account for missing data; analyses were performed with the data and sample sizes available in the medical records. Analyses were performed using R Studio version 2022.07.02 (Posit Software, Boston, MA), with alpha set to 0.05.
Results
Patient demographics & surgical information
We identified 88 patients who underwent ACDF with the anchored spacer during the study period. After applying exclusion criteria, we excluded 24 patients who had prior ACDF surgery, 1 patient who received anterior plating, 2 patients whose surgery was conducted for trauma, and 15 patients without at least 90 days of postoperative follow-up for a final cohort of 46 patients. The cohort selection process is shown in Figure 2.

The study cohort is described in Table 1. Patients underwent surgery at an average of 59.7 years old, and 19 (41.3%) were male. Common comorbidities included hypertension (22, 47.8%) and hyperlipidemia (16, 34.8%). Eight patients (17.4%) were documented as active smokers during preoperative evaluation. Nineteen patients (41.3%) of patients underwent 2-level surgery, 14 (30.4%) had 3-level surgery, and 12 (26.1%) had 1-level surgery. The most common indication for surgery was myelopathy (35, 76.1%). During surgery, the vast majority of patients received intraoperative intravenous (IV) steroids (n=39, 84.8%). A single patient was found to have undergone a 4-level ACDF. This patient was excluded from additional statistical analysis due to a lack of adequate sample size for 4-level ACDF patients, leading to a final cohort of 45 patients for statistical analysis of outcomes (Figure 2).
Table 1
| Characteristic | Statistic |
|---|---|
| Age (years) at surgery, mean ± SD | 59.7±11.5 |
| Male, n (%) | 19 (41.3) |
| BMI (kg/m2) at surgery, mean ± SD | 30.3±6.5 |
| Comorbidities, n (%) | |
| Hypertension | 22 (47.8) |
| Hyperlipidemia | 16 (34.8) |
| Diabetes mellitus | 10 (21.7) |
| Heart disease (CAD, prior MI) | 7 (15.2) |
| Smoking prior to surgery | 8 (17.4) |
| Number of surgical levels, n (%) | |
| 1 | 12 (26.1) |
| 2 | 19 (41.3) |
| 3 | 14 (30.4) |
| 4 (excluded from further analysis) | 1 (2.2) |
| Anatomic levels of surgery—not mutually exclusive, n (%) | |
| C3–4 | 12 (26.1) |
| C4–5 | 25 (54.3) |
| C5–6 | 35 (76.1) |
| C6–7 | 18 (39.1) |
| C7–T1 | 1 (2.2) |
| Received intravenous intraoperative steroids, n (%) | 39 (84.8) |
| Surgical duration (hours), mean ± SD | 3.5±1.6 |
| For 1 level surgeries | 2.6±0.9 |
| For 2 level surgeries | 2.8±0.6 |
| For 3 level surgeries | 5.1±1.8 |
| For 4 level surgeries | 6.2±1 |
| Length of stay in days | |
| Mean ± SD | 2.3±1.5 |
| Median [IQR] | 2 [1–3] |
| Indication for surgery/diagnosis—not mutually exclusive, n (%) | |
| Cervical radiculopathy | 17 (37.0) |
| Cervical myelopathy | 35 (76.1) |
| Neck pain | 7 (15.2) |
| Postoperative follow-up (days) | |
| Mean ± SD | 390±282 |
| Median [IQR] | 303 [196–534] |
SD, standard deviation; BMI, body mass index; CAD, coronary artery disease; MI, myocardial infarction; IQR, interquartile range.
Clinical outcomes
In regard to dysphagia, 17.8% of patients (8/45) developed immediate postoperative dysphagia, of which 15.6% (7/40) experienced sustained dysphagia at 6 weeks after surgery, 13.3 (6/28) at 3 months after surgery, and 2.2% (1/26) at 6 months after surgery. No patients with 1 year of follow-up continued to have dysphagia, and only 2 of the 45 patients (4.4%) reported dysphagia severe enough to necessitate referrals for a video-fluoroscopic/barium swallow study (Table 2).
Table 2
| Outcome | Values |
|---|---|
| Postoperative dysphagia | |
| Immediately postoperative | 8/45 (17.8%) |
| 1-level ACDF | 0 |
| 2-level ACDF | 3 |
| 3-level ACDF | 5 |
| 6 weeks postoperative | 7/40 (15.6%) |
| 1-level ACDF | 0 |
| 2-level ACDF | 2 |
| 3-level ACDF | 5 |
| 3 months postoperative | 6/28 (13.3%) |
| 1-level ACDF | 0 |
| 2-level ACDF | 1 |
| 3-level ACDF | 5 |
| 6 months postoperative | 1/26 (2.2%) |
| 1-level ACDF | 0 |
| 2-level ACDF | 0 |
| 3-level ACDF | 1 |
| 1 year | 0/19 |
| Referred for barium swallow study at any timepoint | 2/45 (4.4%) |
| Intraoperative complication | 3 (6.6%) |
| Loss of MEPs | 2 |
| Dural tear | 1 |
| Postoperative complication | 7 (15.6%) |
| Temporary weakness/neurological deficit after surgery | 3 |
| Minor wound infection—no reoperation required | 2 |
| Hardware complication (shim backout, required reoperation) | 1 |
| UTI | 1 |
| 90-day ED visit | 5 (11.1%) |
| Excessive upper extremity pain | 2 |
| Postoperative nausea & vomiting | 1 |
| Upper extremity paresthesia | 1 |
| Dysphagia and upper extremity paresthesia | 1 |
| 90-day readmission | 2 (4.4%) |
| Upper extremity paresthesia | 1 |
| Excessive upper extremity pain | 1 |
| Reoperation | 7 (15.6%) |
| Adjacent segment disease | 5 |
| Recurrent radiculopathy, foraminotomies performed | 1 |
| Hardware failure | 1 |
| Time to reoperation, days | |
| Mean ± SD | 653±479 |
| Mean [IQR] | 572 [463–761] |
| Range | 1–1,547 |
| Annual incidence of ASD | |
| Overall | 5/45 (11.1%) |
| Year 1 | 0/45 |
| Year 2 | 3/17 (17.6%) |
| Year 3 | 1/5 (20%) |
| Year 4 | 0/1 |
| Year 5 | 1/1 (100%) |
ACDF, anterior cervical discectomy and fusion; MEP, motor endplate potential; UTI, urinary tract infection; ED, emergency department; ASD, adjacent segment disease; SD, standard deviation; IQR, interquartile range.
Three (6.6%) patients had intraoperative complications, 7 (15.6%) had postoperative complications, 5 (11.1%) had postoperative ED visits, 2 (4.4%) had readmissions, and 7 (15.6%) had a reoperation, of which 5 were for ASD. No patients had ASD requiring reoperation during the first year after surgery. At the 2nd year after surgery, 3/17 (17.6% patients with long enough follow-up) required reoperation for ASD, and during the 3rd year after surgery, 1/5 patients (20.0%) required reoperation for ASD. Among those with ASD, the initial surgery was performed at C5–6 for 2 patients, C4–6 for 1 patient, C5–7 for one patient, and C3–6 for one patient. Four of these patients were reoperated below the original surgical segment at C6–7. One patient was reoperated above the original segment at C4–5. These outcomes are detailed in Table 2.
Radiographic outcomes
Radiographic outcomes are shown in Tables 3-5. Changes in disc height were calculated among patients with repeated imaging. Preoperatively to immediately post-surgery, anterior disc height increased by an average of 6.2 mm or 227% and posterior disc height increased by 4.1 mm or 149%. Postoperatively to 6 weeks after surgery, anterior disc height decreased by an average of 1.4 mm or 15% and posterior disc height decreased by 1.4 mm or 19%. From 6 weeks to 3 months after surgery, anterior disc height decreased by an average of 0.5 mm or 6% and posterior disc height decreased by 0.5 mm or 11%. At 6 weeks after surgery the number of measured segments that showed subsidence of >3 mm was 2/25 (8.0%) and at 3 months post-operative it was 1/24 (4.2%). This subsidence occurred in 2/12 (16.6%) patients at 6 weeks and 1/11 (11.1%) patients at 3 months. Details on disc height and subsidence are shown in Table 3.
Table 3
| Characteristic | Preoperative to postoperative | Postoperative to 6 weeks | 6 weeks to 3 months |
|---|---|---|---|
| Total No. of segments | 22 | 25 | 24 |
| Total No. of patients | 10 | 12 | 11 |
| Anterior disc height, mean ± SD | |||
| mm change | 6.2±1.7 | −1.4±1.0 | −0.5±1.1 |
| % change | 227±151 | −15±11 | −6±13 |
| Posterior disc height, mean ± SD | |||
| mm change | 4.1±0.9 | −1.4±1.0 | −0.5±1.1 |
| % change | 149±59 | −19±12 | −11±20 |
| Significant subsidence | |||
| No. of segments | N/A | 2 | 1 |
| No. of patients | N/A | 2 | 1 |
SD, standard deviation; N/A, not applicable.
Table 4
| Radiographic measurement | Preoperative | 6 weeks | 3 months |
|---|---|---|---|
| Global lordosis | |||
| Mean ± SD, degrees | 10.4±9.3 | 10.5±7.8 | 8.9±7.9 |
| n | 34 | 37 | 15 |
| Segmental lordosis | |||
| Mean ± SD, degrees | 6.9±7.3 | 5.9±6.6 | 7.0±5.9 |
| n | 33 | 38 | 15 |
SD, standard deviation.
Table 5
| Radiographic measurement | P values | ||
|---|---|---|---|
| Preoperative vs. 6 weeks | Preoperative vs. 3 months | 6 weeks vs. 3 months |
|
| Global lordosis | 0.94 | 0.56 | 0.50 |
| Segmental lordosis | 0.53 | 0.98 | 0.56 |
Preoperatively, patients had an average global lordosis of 10.4°±9.3° (n=34) and segmental lordosis of 6.9°±7.3° (n=33). Global and segmental lordosis, respectively, were 10.5°±7.8° (n=37) and 5.9°±6.6° (n=38) at 6 weeks, and 8.9°±7.9° (n=15) and 7.0°±5.9° (n=15) at 3 months (Table 4). Neither global nor segmental lordosis changed significantly between timepoints (Table 5).
At 6 weeks after surgery, 11/38 patients (28.9%) achieved radiographic fusion of all surgical segments. The fusion rate increased to 18/23 (78.3%) at 6 months and 18/18 (100%) at 1 year (Table 6).
Table 6
| Timepoint | Patients with radiographic fusion of all operative segments |
|---|---|
| 6 weeks postoperative | 11/38 (28.9%) |
| 3 months postoperative | 6/16 (37.5%) |
| 6 months postoperative | 18/23 (78.3%) |
| 9 months postoperative | 10/13 (76.9%) |
| 1 year postoperative | 18/18 (100%) |
Case example
Preoperative examination
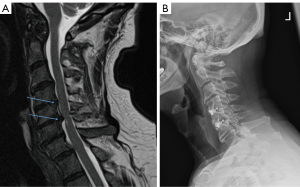
A 70-year-old male presented to clinic with 3 months of neck pain after falling while walking up stairs and hitting his head and neck. He reported significant pain that worsened when looking downward and his symptoms improved minimally after physical therapy. He initially reported no clumsiness or weakness of upper or lower extremities, and strength was 5/5 in all extremities upon examination at the first appointment. Three weeks after initial evaluation, at a follow-up visit 2 weeks prior to surgery, the patient noted increased neck pain, felt significant issues with balance, and felt subjective weakness in the arms and legs. Cervical MRI taken 2 months prior to surgery revealed significant central disc herniation at C4–5 and C5–6 (Figure 3A). Electromyography and nerve conduction study conducted 2 weeks prior to surgery revealed a chronic bilateral C5–6 radiculopathy. The decision was made to proceed with C4–6 ACDF with ISa.
Surgery
The patient underwent C4–6 ACDF with neuromonitoring. An anterior approach was used to dissect open the neck to the vertebral bodies. The C4–5 disc space was exposed, disc removed using curettes & rongeurs, and osteophytes removed using drills. The posterior longitudinal ligament was removed, and dura identified. The ISa spacer was filled with allograft and autograft, placed into the disc space, and secured via anchors hammered into the bodies of C4 and C5. This procedure was repeated at the C5–6 disc space. The patient was given a dose of 10 mg IV dexamethasone during surgery. The surgery was completed in 187 minutes (3.11 hours) without intraoperative or postoperative complications and blood loss was minimal.
Postoperative course
The patient was transferred to a neurosurgery stepdown unit and began ambulating on the day of surgery. The patient achieved good pain control, was able to tolerate a diet, and was able to ambulate independently, thus was discharged home on postop day 1. The patient experienced no immediate postoperative dysphagia, and no dysphagia developed during follow-up. The patient recovered well and presented to clinic with resolution of preoperative neck pain and imbalance, which was maintained at a year after surgery. The patient achieved adequate bony fusion at both operative segments (Figure 3B) and has not required any revision surgery with over 2 years of follow-up.
Discussion
This case series presents a set of patients who received ACDF surgeries using a stand-alone anchored spacer system (ISa). This type of surgery can be considered minimally invasive as it makes use of anchors that require minimum disruption of the neck’s anatomy as compared to using screws which would require additional steps for screw preparation and angled instruments for fixation. The ISa spacers described here use anchors that can be inserted in a compact working window due to the use of a streamlined double barrel implant inserter. This inserter allows for reliable and convenient placement of the implant and its anchors (Figure 1). We evaluated the postoperative course of patients, focusing on postoperative dysphagia, complications, readmission, reoperation, and radiographic measures, and found that rates of negative clinical outcomes were comparable or lower than seen in studies analyzing traditional plate-cage systems.
Dysphagia is a common development after ACDF, affecting anywhere from 10–50% of those who undergo the procedure (4,16,17). The proportion of patients with dysphagia in our study decreased over time, suggesting gradual resolution. Dysphagia also only affected those with 2 or 3 level surgeries, which is consistent with literature suggesting that more operative segments increase the risk for dysphagia (16,17). Systematic reviews comparing anchored, stand-alone spacers to traditional plated ACDF showed that plated systems have a significantly higher risk of dysphagia compared to anchored, stand-alone systems like the implant we describe here (4,18). While most of our patients received preoperative steroids, a systematic review on steroid use in ACDF found that there are no standardized steroid use protocols and that steroids have conflicting outcomes on postoperative dysphagia in different studies (6). An appropriately powered prospective trial will be needed to evaluate steroid use for the prevention of dysphagia.
Dysphagia rates with plate-cage systems have been reported at 25–70% immediately postoperatively, decreasing to 25–27% at 3 months after surgery and 4–22% 6 months after surgery (18). Other low-profile or plate-less cages have reported dysphagia rates of 22–57% immediate after surgery, 4–7% 3 months after surgery, and 0–4% 6 months after surgery (4,18).
This study showed a much lower immediate dysphagia rate of 17.8% and 6-month dysphagia rate of only 2.2%. A potential driver of lower dysphagia in our stand-alone cage patients compared to the literature is the lack of a plate that can irritate the esophagus and the integrated anchoring system. This system allows for the surgery to be carried with less traction on the esophagus as the entire operative length does not need to be exposed to screw in a multi-level plate. Instead, cages can be placed and secured at each vertebral segment with minimal retraction on the surrounding soft tissue.
Reported complication rates for ACDF range from 13% to 20% (16,19). Nationally, the readmission rate for ACDF is about 8% whereas this study showed only 4.4% (20,21). Additionally, the average length of stay for plated ACDF patients is about 2 days (22). This is similar to the results of this study which show average length of stay to be 2.3 days. The complications in our patients are commonly seen with ACDF, including infection, worsening myelopathy, dural tears and hardware complications (16,23). Only 1 patient had a hardware issue and was promptly reoperated on a day after the initial procedure. While 5 patients in this cohort (11.1%) reported to the ED within 90 days of surgery (two for pain, one for dysphagia, one for paresthesia, and one for postoperative nausea and vomiting), only 2 (4.4%) patients were readmitted to the hospital.
Among patients with follow up, the rate of ASD was zero during the first year of follow-up but increased to around 18% during subsequent years. Hilibrand et al. studied symptomatic ASD in anterior cervical surgeries and found an overall rate of ASD of 14.2% among 409 patients, with 2–3% being affected yearly and 13.5% of patients developing the disease within 5 years of surgery (24). Only 27 (6.6%) of patients in that study underwent reoperation, which is similar to reported rates of ACDF reoperation in larger meta-analyses (25) and lower than 11.1% annual ASD rate seen in this study. However, our study is severely limited by its lower sample size and shorter follow-up rate among patients, so future studies will need to be expanded to accurately evaluate symptomatic ASD with ISa.
The patients in our study did not show any significant changes in lordosis, and the average segmental and global lordosis angles were comparable at 3 months postoperatively compared to preoperatively. At long term follow-up 6 or more months after surgery, most patients had achieved full radiographic fusion of all operative segments. For patients who had measurable disc height at multiple timepoints, about 17% of them had significant subsidence over 3 mm, consisting of about 8% of operative segments at 6 weeks after surgery.
A number of studies have analyzed the radiographic differences between traditional plated ACDF systems and stand-alone spacers. Overall, the rates of fusion achieved in this cohort was 100% at 1 year which is comparable to rates of over 90% reported for both stand-alone and plated ACDF systems at 1 year after surgery (26,27). The 100% fusion rate might be attributed to the engraved surfaces on the ISa spacers described here, which were designed to encourage cellular activity at the interface of the spacer and the end-plates of the vertebral body. Other studies have shown that rates of subsidence in stand-alone spacer systems range from 45–70% (28,29) and are generally higher than plated systems, which have subsidence rates of 20–45% (12,29,30). However, there doesn’t seem to be a clear consensus as to whether spacer subsidence affects clinical outcomes. Some studies report that even in patients with spacer subsidence >3 mm, there is no association with pain, pseudarthrosis, or healthcare-related quality of life measures (13,31), while another found that subsidence is associated with increased pain after surgery, but not to any clinically significant degree (32).
Limitations & future directions
This study contains several limitations that could be addressed by future studies. Firstly, this study is a single-surgeon, retrospective study and does not contain a control or comparison group. Thus, we can only speculate on the benefits of this stand-alone spacer system based on results reported elsewhere in the literature. Secondly, the sample size is relatively small at 40, and not all patients had long-term follow up or imaging at every timepoint we analyzed.
A key limitation is that dysphagia, as evaluated in this study, was retrospectively collected through review of medical record reports. However, this has been shown to lead to under-reporting of true dysphagia (33,34) and is not as granular as certain dysphagia scales such as the SWAL-QOL (35) or EAT-10 (36). These should be addressed in future prospective studies that incorporate these scales in a standardized method by collecting them from patients at each planned timepoint.
Additionally, due to artifact from the titanium implant, measuring disc height for all segments at all timepoints proved difficult and would require further study to make definite conclusions about subsidence. Finally, we were limited in our clinical outcomes and unable to provide pain outcomes with scores such as the visual analog scale (VAS) or neck disability index (NDI), because these were not standardized for entry into the electronic medical record. The results from this case series should serve as the basis of a prospective, randomized clinical trial comparing this spacer to other stand-alone spacer systems and traditional plated ACDF systems. Based on rough incidence estimates from our study and the literature cited above, a study powered to detect a dysphagia rate of 18% stand-alone vs. 30% plate-cage would require 396 total patients in a 1:1 enrollment with an alpha of 0.05 and power of 0.80.
Conclusions
This study contains a case series of patients who underwent 1–4 level ACDF with ISa. It showed that immediate post-operative and 6-month post-operative dysphagia rate with ISa was lower than literature showing patients implanted with plated spacers. Radiographic outcomes in this cohort are similar to those reported elsewhere in the literature, with the highlight being 100% fusion rates at 1 year follow up. The rates of complications were similar to previous studies, but patients in this cohort were readmitted to the hospital at a lower rate than is seen nationally. This study’s results suggest that the ISa has some approach-related benefits and is a viable alternative to traditional cage plate systems. The results from this study should be followed up with larger controlled trials, directly comparing this spacer with other ones used in ACDF surgery and utilizing established outcomes scales to more accurately measure dysphagia and radiographic outcomes.
Acknowledgments
Data from this manuscript were presented at the 2023 AANS/CNS Spine Summit in March 2023.
Funding: This study was made possible in part by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-24-32/rc
Data Sharing Statement: Available at https://jss.amegroups.com/article/view/10.21037/jss-24-32/dss
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-24-32/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-24-32/coif). K.D.T. serves as an unpaid editorial board member of Journal of Spine Surgery from December 2022 to November 2024. V.V. reports research finding through a medical student training grant was given by the NIH (No. TL1 TR002555). All authors report that this study was supported by a grant from Globus, Inc. to present data at the 2023 Spine Summit conference. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board (IRB) of Duke University Medical Center under Pro00090408. Due to the retrospective nature of this study, informed consent was deemed not necessary.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Iyer S, Kim HJ. Cervical radiculopathy. Curr Rev Musculoskelet Med 2016;9:272-80. [Crossref] [PubMed]
- Hofstetter CP, Kesavabhotla K, Boockvar JA. Zero-profile Anchored Spacer Reduces Rate of Dysphagia Compared With ACDF With Anterior Plating. J Spinal Disord Tech 2015;28:E284-90. [Crossref] [PubMed]
- Khalifeh K, Faulkner JE, Hara J, et al. A Retrospective Evaluation and Review of Outcomes for Single- and Multilevel ACDF With a Zero-Profile Stand-Alone Cage Device With Integrated Instrumentation. Cureus 2021;13:e14283. [Crossref] [PubMed]
- Gabr MA, Touko E, Yadav AP, et al. Improved Dysphagia Outcomes in Anchored Spacers Versus Plate-Screw Systems in Anterior Cervical Discectomy and Fusion: A Systematic Review. Global Spine J 2020;10:1057-65. [Crossref] [PubMed]
- Segebarth B, Datta JC, Darden B, et al. Incidence of dysphagia comparing cervical arthroplasty and ACDF. SAS J 2010;4:3-8. [Crossref] [PubMed]
- Siasios I, Fountas K, Dimopoulos V, et al. The role of steroid administration in the management of dysphagia in anterior cervical procedures. Neurosurg Rev 2018;41:47-53. [Crossref] [PubMed]
- Jeyamohan SB, Kenning TJ, Petronis KA, et al. Effect of steroid use in anterior cervical discectomy and fusion: a randomized controlled trial. J Neurosurg Spine 2015;23:137-43. [Crossref] [PubMed]
- Nam TW, Lee DH, Shin JK, et al. Effect of intravenous dexamethasone on prevertebral soft tissue swelling after anterior cervical discectomy and fusion. Acta Orthop Belg 2013;79:211-5. [PubMed]
- Wang TY, Mehta VA, Sankey EW, et al. Characterization and rate of symptomatic adjacent-segment disease after index lateral lumbar interbody fusion: a single-institution, multisurgeon case series with long-term follow-up. J Neurosurg Spine 2021;35:139-46. [Crossref] [PubMed]
- Oshina M, Oshima Y, Tanaka S, et al. Radiological Fusion Criteria of Postoperative Anterior Cervical Discectomy and Fusion: A Systematic Review. Global Spine J 2018;8:739-50. [Crossref] [PubMed]
- Jin ZY, Teng Y, Wang HZ, et al. Comparative Analysis of Cage Subsidence in Anterior Cervical Decompression and Fusion: Zero Profile Anchored Spacer (ROI-C) vs. Conventional Cage and Plate Construct. Front Surg 2021;8:736680. [Crossref] [PubMed]
- Yson SC, Sembrano JN, Santos ER. Comparison of allograft and polyetheretherketone (PEEK) cage subsidence rates in anterior cervical discectomy and fusion (ACDF). J Clin Neurosci 2017;38:118-21. [Crossref] [PubMed]
- Milczynska WM, Ahmad A, Ahmed AI, et al. Does titanium cage subsidence affect clinical outcomes in ACDF surgery? A tertiary centre experience. Ann R Coll Surg Engl 2023;105:378-83. [Crossref] [PubMed]
- Pinter ZW, Mikula A, Shirley M, et al. Allograft Subsidence Decreases Postoperative Segmental Lordosis With Minimal Effect on Global Alignment Following ACDF. Global Spine J 2022;12:1723-30. [Crossref] [PubMed]
- Godlewski B, Bebenek A, Dominiak M, et al. Subsidence following cervical discectomy and implant-to-bone ratio. BMC Musculoskelet Disord 2022;23:750. [Crossref] [PubMed]
- Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976) 2007;32:2310-7. [Crossref] [PubMed]
- Shriver MF, Lewis DJ, Kshettry VR, et al. Dysphagia Rates after Anterior Cervical Diskectomy and Fusion: A Systematic Review and Meta-Analysis. Global Spine J 2017;7:95-103. [Crossref] [PubMed]
- Tong MJ, Xiang GH, He ZL, et al. Zero-Profile Spacer Versus Cage-Plate Construct in Anterior Cervical Diskectomy and Fusion for Multilevel Cervical Spondylotic Myelopathy: Systematic Review and Meta-Analysis. World Neurosurg 2017;104:545-53. [Crossref] [PubMed]
- Epstein NE. A Review of Complication Rates for Anterior Cervical Diskectomy and Fusion (ACDF). Surg Neurol Int 2019;10:100. [Crossref] [PubMed]
- Elsamadicy AA, Ren X, Kemeny H, et al. Independent Associations With 30- and 90-Day Unplanned Readmissions After Elective Lumbar Spine Surgery: A National Trend Analysis of 144 123 Patients. Neurosurgery 2019;84:758-67. [Crossref] [PubMed]
- Taylor BES, Hilden P, Hansen RTB, et al. National Rates, Reasons, and Risk Factors for 30- and 90-Day Readmission and Reoperation Among Patients Undergoing Anterior Cervical Discectomy and Fusion: An Analysis Using the Nationwide Readmissions Database. Spine (Phila Pa 1976) 2021;46:1302-14. [Crossref] [PubMed]
- Sommaruga S, Camara-Quintana J, Patel K, et al. Clinical Outcomes between Stand-Alone Zero-Profile Spacers and Cervical Plate with Cage Fixation for Anterior Cervical Discectomy and Fusion: A Retrospective Analysis of 166 Patients. J Clin Med 2021;10:3076. [Crossref] [PubMed]
- Elsamadicy AA, Koo AB, Lee M, et al. Patient Risk Factors Associated With 30- and 90-Day Readmission After Cervical Discectomy: A Nationwide Readmission Database Study. Clin Spine Surg 2020;33:E434-41. [Crossref] [PubMed]
- Hilibrand AS, Carlson GD, Palumbo MA, et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am 1999;81:519-28. [Crossref] [PubMed]
- Toci GR, Canseco JA, Patel PD, et al. The Incidence of Adjacent Segment Pathology After Cervical Disc Arthroplasty Compared with Anterior Cervical Discectomy and Fusion: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. World Neurosurg 2022;160:e537-48. [Crossref] [PubMed]
- Scholz M, Onal B, Schleicher P, et al. Two-level ACDF with a zero-profile stand-alone spacer compared to conventional plating: a prospective randomized single-center study. Eur Spine J 2020;29:2814-22. [Crossref] [PubMed]
- De Leo-Vargas RA, Muñoz-Romero I, Mondragón-Soto MG, et al. Locking Stand-Alone Cage Constructs for the Treatment of Cervical Spine Degenerative Disease. Asian Spine J 2019;13:630-7. [Crossref] [PubMed]
- Ryu HS, Han MS, Lee SS, et al. Influence of subsidence after stand-alone anterior cervical discectomy and fusion in patients with degenerative cervical disease: A long-term follow-up study. Medicine (Baltimore) 2022;101:e30673. [Crossref] [PubMed]
- Shin JS, Oh SH, Cho PG. Surgical Outcome of a Zero-profile Device Comparing with Stand-alone Cage and Anterior Cervical Plate with Iliac Bone Graft in the Anterior Cervical Discectomy and Fusion. Korean J Spine 2014;11:169-77. [Crossref] [PubMed]
- Xu J, He Y, Li Y, et al. Incidence of Subsidence of Seven Intervertebral Devices in Anterior Cervical Discectomy and Fusion: A Network Meta-Analysis. World Neurosurg 2020;141:479-489.e4. [Crossref] [PubMed]
- Pinter ZW, Reed R, Townsley SE, et al. Titanium Cervical Cage Subsidence: Postoperative Computed Tomography Analysis Defining Incidence and Associated Risk Factors. Global Spine J 2023;13:1703-15. [Crossref] [PubMed]
- Obermueller T, Wagner A, Kogler L, et al. Radiographic measurements of cervical alignment, fusion and subsidence after ACDF surgery and their impact on clinical outcome. Acta Neurochir (Wien) 2020;162:89-99. [Crossref] [PubMed]
- Edwards CC 2nd, Karpitskaya Y, Cha C, et al. Accurate identification of adverse outcomes after cervical spine surgery. J Bone Joint Surg Am 2004;86:251-6. [Crossref] [PubMed]
- Molfenter SM, Amin MR, Balou M, et al. A scoping review of the methods used to capture dysphagia after anterior cervical discectomy and fusion: the need for a paradigm shift. Eur Spine J 2023;32:969-76. [Crossref] [PubMed]
- McHorney CA, Robbins J, Lomax K, et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia 2002;17:97-114. [Crossref] [PubMed]
- Zhang PP, Yuan Y, Lu DZ, et al. Diagnostic Accuracy of the Eating Assessment Tool-10 (EAT-10) in Screening Dysphagia: A Systematic Review and Meta-Analysis. Dysphagia 2023;38:145-58. [Crossref] [PubMed]


