
Electromagnetic navigation guided tailored lamino-pedicular intralesional marginal resection of recurrent sacral osteoblastoma: a case report
Highlight box
Key findings
• Electromagnetic navigation (EMN) guided intralesional marginal resection of spinal osteoblastoma demonstrated significant advancement in spinal tumor surgery. EMN allowed for precise tumor localization and tailored resection, resulting in complete tumor removal and symptom resolution during a 2-year follow-up period.
What is known and what is new?
• Spinal osteoblastomas pose management challenges due to their potential for recurrence and proximity to critical structures. While traditional surgical approaches exist, EMN-guided surgery represents an innovative solution. This manuscript adds to existing knowledge by demonstrating the efficacy of EMN in achieving precise tumor localization and tailored resection in cases of spinal osteoblastomas.
What is the implication, and what should change now?
• The effectiveness of EMN-guided surgery suggests its potential as a valuable tool in spinal tumor resection procedures. Clinicians should consider incorporating EMN into routine practice to enhance precision and improve patient outcomes. Further research and reporting will help confirm the efficacy of EMN and refine its application in the treatment of spinal tumors, ultimately benefiting patients.
Introduction
Spinal osteoblastomas are rare primary osteogenic neoplasms with an affinity for the posterior elements, typically involving the lumbar spine and, least commonly, affecting the sacral region, whereby extension from the posterior elements into the vertebral body is not uncommon (1). Considering their vast nature of presentation and high recurrence rate, treatment options for symptomatic spinal osteoblastomas vary, ranging from percutaneous minimally invasive interventions to surgical en bloc resection (1,2).
Modern modalities of computer-assisted orthopedic surgery (CAOS) enable the preoperative patient-personalized three-dimensional (3D) planning and may facilitate surgical treatment for locally aggressive and recurrent osteoblastomas, thereby increasing the safety and precision of challenging surgical resections (3).
We present a case of electromagnetic navigation (EMN) guided tailored lamino-pedicular intralesional marginal resection of recurrent S1 osteoblastoma. We present this article in accordance with the CARE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-24-58/rc).
Case presentation
A disease-free 17-year-old female was referred to our institution due to a persistent 9-month history of sacral pain, without associated trauma or infection. She reported pain during daily activities, resting and night-time, necessitating the regular use of non-opioid analgesics. No neurologic impairment or disturbances in the musculoskeletal system were observed. The patient’s basic laboratory findings were within normal range.
We performed an advanced imaging [computed tomography (CT) scan, magnetic resonance imaging (MRI), bone scintigraphy, and single-photon emission CT (SPECT)/CT], which showed a round osteolytic lesion approximately 19 mm in diameter with central calcification located in the left lamina and pedicle of the S1 vertebra, surrounded by a wide zone of reactive sclerosis—Enneking stage 2 (4) (Figure 1). The case was discussed by a multidisciplinary board, leading to the consensus that the lesion was an osteoid osteoma, obviating the necessity for a biopsy. Due to anatomical proximity of the sacral nerves, radiofrequency ablation was contraindicated. We performed a surgical intralesional resection with a left-sided hemilaminectomy of L5 and S1, guided by a gamma probe after preoperative intravenous technetium contrast administration. Hemilaminectomy of L5 was primarily not planned, however it was performed as a safety precaution due to the gamma probe signaling pattern upon the surgical access. The postoperative course was uneventful, and the patient was discharged 2 days after surgery. The pathohistological analysis of the biopsy revealed that the lesion was not consistent with osteoid osteoma but rather diagnosed as osteoblastoma. Given this information, we planned a close clinical follow-up of the patient.
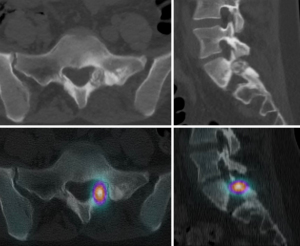
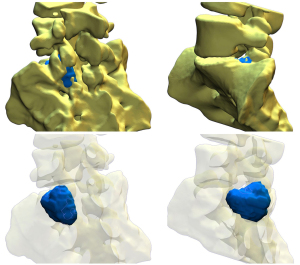
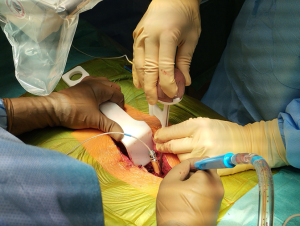
During the regular follow-up, 1 year after the surgery, the patient reported a recurrence of sacral pain, similar to the index issues. Advanced follow-up imaging (MRI, CT, bone scintigraphy, and SPECT-CT) was performed. The images showed a recurrence of osteoblastoma—a round osteolytic lesion with central calcification approximately 15 mm in diameter located in the left pedicle and lamina of the S1 vertebra surrounded by a zone of reactive sclerosis—Enneking stage 2 (Figure 2). Given the clinical and radiological findings, we once again opted for an intralesional resection, this time using the assistance of an EMN system (Guiding Star, Ekliptik d.o.o., Ljubljana, Slovenia). The images of CT, MRI, and SPECT-CT scans were preoperatively imported into the EBS medical software (Ekliptik d.o.o.) to generate a virtual 3D model of the patient’s lumbosacral junction visualizing the exact location of the recurrent osteoblastoma (Figure 3). With the help of a software specialist, the surgeon planned the desired amount of resection and determined the appropriate margin. To account for potential EMN error, the margin distance was established at 2 mm from the tumor. The EMN system used in this case works on the principle of surface-based registration. During surgery, following an open posterior midline approach to the lumbosacral junction, an EMN reference sensor was positioned onto the L4 spinous process (Figure 4). Afterward, several reference points were captured on the exposed lumbosacral surface with a designated EMN pointer probe. The digitized surface points were then superimposed on the previously rendered 3D model, allowing for exact intraoperative, real-time visualization of the tumor localization. The tumor resection was performed with a combination of piecemeal resection with a small chisel and a disc rongeur and an employment of a high-speed drill at the borders, with the extension of resection determined preoperatively and confirmed intraoperatively with the assistance of the EMN pointer probe (Figure 5). In addition to the tumor, the S1 spinous process and the whole left S1 pedicle were also removed to allow for better surgical access. Consequently, we performed an instrumented fusion of the L5 and S1 vertebrae with pedicular screws and intracorporeal cage. The postoperative course was again uneventful and the patient was discharged 4 days after the surgery. Pathohistological findings confirmed that the tumor was indeed a recurrence of the osteoblastoma. During the follow-up, 2 years after the second surgery, she remains pain free and neurologically intact without radiological signs of recurrence and/or instability.

All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s legal guardian for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The presented case emphasizes the challenges in diagnostics and management of osteoblastomas of the spine and demonstrates the value of EMN guidance for their treatment.
Osteoid osteomas and osteoblastomas are benign bone tumors with a similar clinical presentation and histological profile. Additionally, radiological findings may exhibit a significant resemblance between the two. The main distinction resides in the potentially higher biological aggressiveness of osteoblastomas, which are typically larger than osteoid osteomas (>2 cm) and are capable of local invasion (1,5,6). Diagnostic inaccuracies are therefore not uncommon.
Treatment options for osteoid osteomas typically encompass less aggressive approaches, with spontaneous resolutions observed in some cases (1,5-7). Surgical management modalities include open excision, CT-guided radiofrequency ablation, and various other minimally invasive approaches such as cryotherapy, laser photocoagulation, and ethanol injection. In contrast, osteoblastomas usually mandate surgical intervention, involving either intralesional or en bloc resection, whereas in select cases, percutaneous radiofrequency or cryoablation techniques may be used (1,5,6). The fully endoscopic approach for osteoblastoma resection offers a potentially safe and effective technique, whereas carbon ion radiotherapy may be recommended for recurrent osteoblastomas following multiple surgeries (8,9).
In benign bone tumor surgery, gamma probe is utilized for precise localization and complete removal of the tumor. It is mainly used for resection of osteoid osteomas. Other authors report good results using this technique (10). Nevertheless, this was not the case in our patient in whom there was a recurrence of osteoblastoma after gamma probe-assisted surgery probably due to insufficient tumor removal.
Some authors suggest the utilization of the Enneking staging system (4) as a guide in determining for appropriate operative treatment of osteoblastomas. They propose performing intralesional resection for stage two osteoblastomas and en bloc resection for stage three osteoblastomas (11). Conversely, based on the results of a retrospective analysis of 50 consecutive cases, Cao et al. suggest that intralesional marginal resection might serve as a suitable therapeutic option for patients diagnosed with stage two and three spinal osteoblastoma (12). Overall recurrence rate for osteoid osteomas and osteoblastomas is relatively high, with some authors reporting recurrence rates of up to 16% following radiofrequency ablation for osteoid osteomas and up to 19% following intralesional resection for osteoblastomas (5,6,11,13,14). In our patient, we have decided for intralesional resection in both instances. Despite the initial diagnostic consideration of osteoid osteoma, the decision for intralesional resection aligns with recommended surgical strategies for the treatment of osteoblastomas. On both occasions, the tumor exhibited Enneking stage 2 characteristics, for which intralesional resection is recommended.
The advantages of different CAOS methods in the treatment of musculoskeletal diseases have been thoroughly recognized in recent years. In line with the reported case herein, they are particularly useful in challenging circumstances with altered anatomy, revisions, deformities and tumor cases, enabling an individual, patient-personalized approach, emphasizing safety and accuracy (3,15). Given the recurrence, specific osteoblastoma location within the lamina and pedicle of the S1 vertebra, and the intricacies of technical access, we opted for an EMN for its meticulous guidance during the removal procedure. This decision was made based on our past experience, where using EMN for benign bone tumor removal proved successful not only in the spine but also in other anatomically challenging areas, such as the hip. To our knowledge, this is the first described case of spinal bone tumor removal using EMN in the literature. Other authors report testing EMN for percutaneous or open transpedicular screw placement with good results (16,17), using it for percutaneous transforaminal endoscopic discectomy (18), bone tumor removal in craniofacial surgery (19,20), femoral and periacetabular osteotomies (21,22), and also for distal locking of femoral intramedullary nails (23).
EMN operates by inducing an electromagnetic field with a field generator placed near the operating site (Figure 4). Instruments equipped with signal coils are detected within this field and transmit their location data to a computer via a wired connection. We used a reference sensor positioned on the L4 spinous process, a pointer probe and a special plastic/wooden pedicle probe. The biggest limitation of the EMN is that the electromagnetic filed can be disrupted by stainless steel or ferromagnetic instruments. Therefore, for proper use of the EMN, plastic or titanium instruments are required. To align the preoperative 3D model with the patient’s spine, several reference points on the exposed lumbosacral surface need to be captured using the pointer probe. The pointer probe is also used to intraoperatively confirm the planned resection margins. With an accuracy of 1 mm (24), its unique strength lies in the real-time intraoperative visualization of preoperatively planned resection margins, especially in bone tumors that do not exhibit a clear margin to the healthy tissue, providing valuable feedback on the thorough neoplasm removal without subjecting the patient to radiation exposure (Figure 5). This enabled us to perform a true tailored lamino-pedicular intralesional marginal resection of the recurrent sacral osteoblastoma, whereby follow-up MRI revealed no recurrence of osteoblastoma after the second surgery.
In comparison to other 3D-navigation guided surgical modalities, the utilization of EMN mitigates the drawbacks of radiation exposure and high cost while preserving accuracy and precision with real-time imaging for intraoperative confirmation of complete tumor extirpation (25). Regardless of navigation system used, accurate intraoperative localization with complete resection is key to preventing recurrence, whereby partial/subtotal excision can bear a 50% recurrence rate (25,26).
Conclusions
The use of EMN in the surgical management of spinal osteoblastomas represents a promising advancement in orthopedic practice. With precise tumor localization, EMN-guided surgery demonstrates potential for improving surgical outcomes and reducing the risk of recurrence. This case underscores the importance of incorporating innovative technologies like EMN into the practice of spinal surgeons to enhance precision and efficacy in tumor resection procedures. Continued research and reporting will further validate the efficacy and refine the application of EMN in routine practice, ultimately benefiting patients with spinal tumors.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-24-58/rc
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-24-58/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-24-58/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s legal guardian for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ariyaratne S, Jenko N, Iyengar KP, et al. Primary Benign Neoplasms of the Spine. Diagnostics (Basel) 2023;13:2006. [Crossref] [PubMed]
- Versteeg AL, Dea N, Boriani S, et al. Surgical management of spinal osteoblastomas. J Neurosurg Spine 2017;27:321-7. [Crossref] [PubMed]
- Landriel F, Albergo JI, Farfalli G, et al. Navigated multiplanar osteotomies for spinal primary bone tumors. Surg Neurol Int 2022;13:58. [Crossref] [PubMed]
- Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res 1986;9-24. [PubMed]
- Atesok KI, Alman BA, Schemitsch EH, et al. Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg 2011;19:678-89. [Crossref] [PubMed]
- Kan P, Schmidt MH. Osteoid osteoma and osteoblastoma of the spine. Neurosurg Clin N Am 2008;19:65-70. [Crossref] [PubMed]
- Tepelenis K, Skandalakis GP, Papathanakos G, et al. Osteoid Osteoma: An Updated Review of Epidemiology, Pathogenesis, Clinical Presentation, Radiological Features, and Treatment Option. In Vivo 2021;35:1929-38. [Crossref] [PubMed]
- Newman WC, Vaynrub M, Bilsky MH, et al. Full endoscopic resection of a lumbar osteoblastoma: technical note. J Neurosurg Spine 2020;33:252-5. [Crossref] [PubMed]
- Honda A, Iizuka Y, Imai R, et al. Recurrent lumbar-origin osteoblastoma treated with multiple surgery and carbon ion radiotherapy: a case report. BMC Musculoskelet Disord 2020;21:321. [Crossref] [PubMed]
- Etchebehere M, Etchebehere EC, Reganin LA, et al. Intraoperative localization of an osteoid-osteoma using a gamma probe. Int Orthop 2004;28:379-83. [Crossref] [PubMed]
- Boriani S, Amendola L, Bandiera S, et al. Staging and treatment of osteoblastoma in the mobile spine: a review of 51 cases. Eur Spine J 2012;21:2003-10. [Crossref] [PubMed]
- Cao S, Chen K, Jiang L, et al. Intralesional Marginal Resection for Osteoblastoma in the Mobile Spine: Experience From a Single Center. Front Surg 2022;9:838235. [Crossref] [PubMed]
- Shields DW, Sohrabi S, Crane EO, et al. Radiofrequency ablation for osteoid osteoma - Recurrence rates and predictive factors. Surgeon 2018;16:156-62. [Crossref] [PubMed]
- Harrop JS, Schmidt MH, Boriani S, et al. Aggressive "benign" primary spine neoplasms: osteoblastoma, aneurysmal bone cyst, and giant cell tumor. Spine (Phila Pa 1976) 2009;34:S39-47. [Crossref] [PubMed]
- Brumat P, Kunšič O, Novak S, et al. The Surgical Treatment of Osteoarthritis. Life (Basel) 2022;12:982. [Crossref] [PubMed]
- von Jako R, Finn MA, Yonemura KS, et al. Minimally invasive percutaneous transpedicular screw fixation: increased accuracy and reduced radiation exposure by means of a novel electromagnetic navigation system. Acta Neurochir (Wien) 2011;153:589-96. [Crossref] [PubMed]
- Hahn P, Oezdemir S, Komp M, et al. A New Electromagnetic Navigation System for Pedicle Screws Placement: A Human Cadaver Study at the Lumbar Spine. PLoS One 2015;10:e0133708. [Crossref] [PubMed]
- Wu B, Wei T, Yao Z, et al. A real-time 3D electromagnetic navigation system for percutaneous transforaminal endoscopic discectomy in patients with lumbar disc herniation: a retrospective study. BMC Musculoskelet Disord 2022;23:57. [Crossref] [PubMed]
- Liu TJ, Ko AT, Tang YB, et al. Clinical Application of Different Surgical Navigation Systems in Complex Craniomaxillofacial Surgery: The Use of Multisurface 3-Dimensional Images and a 2-Plane Reference System. Ann Plast Surg 2016;76:411-9. [Crossref] [PubMed]
- Chen M, Xia N, Dong Q, et al. The Application of Three-Dimensional Technology Combined With Image Navigation in Nasal Skull Base Surgery. J Craniofac Surg 2020;31:2304-9. [Crossref] [PubMed]
- Erdani D, Trebše R, Brumat P. Electromagnetic Navigation Assisted Patient-Personalized Femoral Osteotomy for Acute Correction of Posttraumatic Residual Multiplanar Femoral Deformity with Shortening. Indian J Orthop 2023;57:344-8. [Crossref] [PubMed]
- Mihalič R, Brumat P, Trebše R. Bernese peri-acetabular osteotomy performed with navigation and patient-specific templates is a reproducible and safe procedure. Int Orthop 2021;45:883-9. [Crossref] [PubMed]
- Han B, Shi Z, Fu Y, et al. Comparison of free-hand fluoroscopic guidance and electromagnetic navigation in distal locking of femoral intramedullary nails. Medicine (Baltimore) 2017;96:e7450. [Crossref] [PubMed]
- Stražar K, Kreuh D, Vouk U, et al. Computer navigation during arthroscopic osteochondroplasty in patients with CAM femoroacetabular impingement. J Hip Preserv Surg 2016;3:hnw030. [Crossref]
- Hadgaonkar SR, Katkade SM, Bhilare PD, et al. Less invasive O-arm navigation-guided excision of thoracic extraosseous intraforaminal osteoblastoma: A case report. Surg Neurol Int 2022;13:263. [Crossref] [PubMed]
- Wu M, Xu K, Xie Y, et al. Diagnostic and Management Options of Osteoblastoma in the Spine. Med Sci Monit 2019;25:1362-72. [Crossref] [PubMed]



