
Kyphoplasty in the setting of corynebacterium striatum septicemia with postoperative osteomyelitis requiring salvage vertebrectomy: a case report
Highlight box
Key findings
• Previous diabetic foot ulcers and septicemia with Corynebacterium striatum can lead to kyphoplasty (KP) complications, including post-operative osteomyelitis and paraspinal abscess.
What is known and what is new?
• While KP is generally a safe procedure with minimal complications, the severity of the complications can have morbid and sometimes fatal implications.
• This case adds another mechanism by which KP can go awry. The patient presents with a compression fracture and a history of diabetic foot ulcer, complicated by hematogenous seeding, who underwent KP, resulting in severe osteomyelitis requiring salvage vertebrectomy.
What is the implication, and what should change now?
• Patients who qualify for KP with recent infection should be properly screened for possible infectious seeding and risk of osteomyelitis post-KP. Further research must be conducted to determine proper KP guidelines following septicemia.
Introduction
Background
Kyphoplasty (KP) is a well-established surgical technique used to stabilize vertebral compression fractures (VCFs) with minimal complications and positive surgical outcomes. VCFs, often resulting from osteoporosis, are extremely common with an estimated US incidence of 750,000 cases by 2025 (1). With mild symptoms, VCFs can be treated medically. However, VCFs have been proven to reduce quality of life and increase mortality, both of which can be solved via proper surgical intervention (2,3). Current minimally invasive treatments are percutaneous vertebroplasty (VP) and KP, both of which achieve pain relief and functional recovery. In the inpatient setting, VP and KP have been shown to decrease hospital stay, readmission rates, and long-term mortality relative to medical treatment (4-7). However, there are no clear guidelines regarding complications management of KP, especially in regard to systemic infections.
Infection following a KP procedure is rare; however, there have been several case reports published presenting this complication. These infections are caused by a wide variety of microorganisms, including Staphylococcus aureus, Staphylococcus haemolyticus, Pseudomonas aeruginosa, Candida albicans, Mycobacterium tuberculosis, and Enterococcus faecium (8-10). Many cases also report infectious symptoms, including back pain and elevated inflammatory markers, from which biopsy cultures did not grow any microorganisms (8,11). Nonetheless, the diversity of reported organisms requires different treatments, highlighting the importance of intra-operative culture for identification and drug susceptibility purposes.
When infection does occur following a KP, there is no standard treatment protocol. Treatment depends on the severity and location of the infection, as well as clinical judgement. A few cases have been treated conservatively (11). Wendling et al. describes the cases of two patients who presented 6 and 10 weeks after a KP with suspicion for infection due to severe back pain and elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP); however, biopsy grew no microorganisms on culture (11). Each patient received no further intervention for treatment of the suspected infection, and their symptoms eventually resolved (11).
Conversely, there are many cases described in the literature in which post-KP infection required a salvage operation. Wendling et al. describes the cases of four individuals who required subsequent salvage surgery following post-KP infection with an average time to revision of 12.75 months (11). Shin et al. describes two patients, who presented 2 and 7 months post-op, with severe back pain and elevated ESR and CRP (8). One patient required anterior corpectomy and reconstruction with a mesh cage and posterior fusion. The other patient required anterior corpectomy and anterior reconstruction with autogenous iliac bone graft (AIBG). Following the salvage surgeries, both patients had growth from the intra-operative culture, were treated with appropriate antibiotics, and had no further complications (8). Failure to treat these post-operative infections properly can result in serious long-term immobility, chronic pain, sepsis, and even death.
The origins of infection are often unknown. They are often assumed to be from skin flora; however, some cases have reported KP with concurrent infection, suggesting a potentially different mechanism of action. For example, one case describes a patient who underwent KP with an active urinary tract infection with cultures positive for Pseudomonas aeruginosa and Candida albicans (8). Two months later, the patient presented with severe back pain and elevated ESR and CRP. Magnetic resonance imaging (MRI) showed a paravertebral abscess for which she underwent a salvage operation of posterior fusion followed by anterior corpectomy and anterior reconstruction with AIBG. Intraoperative cultures did not grow any organisms. Post-operative intravenous antibiotics were continued for 6 weeks, followed by 2 months of oral antibiotics with no further complications (8). While the origin of this post-KP infection is inconclusive, performing a KP in the setting of active infection may provide an addition route for microbiota to seed the surgical site.
Ivo et al. describes another case of KP in the setting of systemic infection, further complicated by postoperative infection (9). A patient with chronic obstructive pulmonary disease and other comorbidities was started on ciprofloxacin due to abnormal bloodwork and pulmonary infiltrates on imaging. After resolution of the infection two weeks later, the patient underwent KP with cephazolin antibiotic prophylaxis. Two weeks after the operation, the patient presented with elevated CRP and an MRI showing a paraspinal abscess and spondylitis, despite negative blood cultures. Ceftriaxone and flucloxacillin was initiated followed by laminectomy, resection of intervertebral discs, corpectomy, and resection of infected tissue. Intraoperative biopsy grew Mycobacterium tuberculosis and Enterococcus faecium. Despite antibiotic treatment, the patient passed away from bacteremia and septic multiple organ failure (9). In this case, KP in the setting the systemic illness provided a route for microbiota to seed the surgical site, resulting in a fatal complication. Overall, while KP is a relatively safe procedure, its infectious risk must not be overlooked. Failure of preoperative diligence can result in severe and fatal consequences. This case describes a treatment course that, to the best of our knowledge, has not been described in literature before. Infection following KP only occurs in approximately 0.11% of cases, and this case exhibited persisting symptoms despite antibiotic treatment, which required subsequent operation many months later (12). This report hopes to identify precautions and management strategies to better prevent complications in future similar cases.
Objective
Our goal is to present a case report of the complications that can follow KP performed in the setting of systemic infection. Specifically, we describe the rare case of a Corynebacterium striatum and Pseudomonas aeruginosa postoperative infection, adding to the list of microorganisms that may contribute an infectious risk to performing a KP. We present this case in accordance with the CARE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-24-31/rc).
Case presentation
We present the case of a 59-year-old male who presented with an infected diabetic ulcer of the right foot on June 9, 2022 (Figure 1). The patient’s medical history consists of type II diabetes mellitus (hemoglobin A1c: 6.3% on February 2, 2022), diagnosed in 1992, complicated by diabetic nephropathy and immunoglobulin A (IgA) nephropathy. Upon presentation, the patient had an ESR of 97 mm/h and white blood cell count (WBC) of 10.7 G/L. The wound was swabbed, and deep wound cultures were positive for Corynebacterium striatum and Enterococcus faecium. An MRI was completed showing possible osteomyelitis versus reactive osteitis. The ulcer was subsequently debrided followed by treatment course of intravenous (IV) vancomycin and piperacillin/tazobactam. The patient was discharged after 4 days with a prescribed regimen of oral piperacillin/tazobactam for 14 days. The patient was readmitted on June 22, 2022 due to acute kidney injury with concurrent pneumonitis and was started on IV vancomycin, which was then changed to oral levofloxacin and clindamycin. The patient was discharged on June 25, 2022 with a 5-day course of clindamycin and levofloxacin. On August 18, 2022, the patient developed dizziness, shortness of breath, and acute pain in his lower back which began after bending down to pick up a pair of shoes. Labs showed an increased WBC of 16.2 G/L and blood cultures positive for Corynebacterium striatum and Pseudomonas aeruginosa infection, consistent with septicemia. The infection was treated with IV cefepime and clindamycin, and a neurosurgical consult was called to address his back pain. A computed tomography (CT) scan of the abdomen was completed to rule out the kidney stones and an acute L1 compression fracture was noted. Further, an MRI on August 25, 2022 confirmed the acute L1 compression fracture in addition to a chronic compression fracture in L2, which was not surgically treated (Figure 2). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.

On September 1, 2022, 13 days after the diagnosis of septicemia, an L1 KP was performed by an interventionalist to treat the acute compression fracture (Figure 3). No antibiotics were utilized within the bone cement and the procedure was considered uncomplicated, and the patient was discharged that same day. Four months later, on January 5, 2023, the patient returned with increasingly severe back pain, spinal tenderness, kyphosis, and ambulatory difficulties due to pain. Physical exam revealed no focal neurologic deficits, but present were signs of chronic diabetic neuropathy in his lower extremities. The patient was referred to an orthopedic spine surgeon. In-office X-ray was performed and showed complete collapse of the L1 vertebrae, loss of surrounding vertebral body and disc space, and increasing kyphosis from T12–L2 with a sagittal alignment angle of 47.5 degrees. MRI and lab work was completed on January 10, 2023 confirming apparent L1 vertebral osteomyelitis with psoas abscess (Figures 4,5). A CT guided biopsy was performed of L1–L2 disc space with subsequent psoas abscess fluid collection for cultures, sensitivity, and drainage. Results of cultures confirmed infection with vancomycin-sensitive Corynebacterium striatum. A course of vancomycin was begun and changed to daptomycin as per infectious disease. The patient’s back pain subsequently improved, and he elected not to have surgical reconstruction.


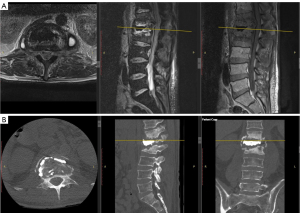
Over the next 5 months, the patient noted a repeated occurrence of severe back pain and diminished ambulatory ability. Repeat standing X-rays demonstrated increasing kyphosis, while MRI and CT revealed recurrence of bilateral psoas fluid collection and further vertebral collapse from T12–L2 (Figure 6A,6B). Repeat aspiration and drainage of psoas abscess showed no recurrent growth of bacteria. Due to continued incapacitating pain, progressive kyphosis from vertebral body infectious destruction, and diminished ambulatory ability, it was determined that the integrity of spinal stability was lost. In order to improve alignment and stability of the thoracolumbar region, the patient underwent L1 vertebrectomy via a retroperitoneal approach with reconstruction and fusion from T12–L2 on August 22, 2023. Reconstruction utilized a femoral shaft allograft with administration of bone morphogenic protein (BMP) followed by T9–L4 posterior stabilization and fusion with pedicle screws. Intraoperative cultures of cement, disc material, and fluid remained negative. The patient’s hospital course was uneventful despite his various comorbidities. After 10 days, on September 1, 2023, the patient was transferred to the rehabilitation unit to continue improving ambulation and activities of daily living. Follow-up X-ray imaging (Figure 7A,7B) revealed an improved sagittal alignment angle of 21.5 degrees from T12–L2 with early incorporation of fusion. Since the operation, the patient has shown significant improvement in alignment, ambulatory ability, and pain relief with no neurologic deficits. At 1-year post-operation, the patient will be recommended for posterior spinal hardware removal to prevent any possible development of recurrent infection within the hardware. The overall treatment course is described in Figure 8.

Patient perspective
A patient interview was conducted to gain insight into his perspective. At his point of maximal pain and prior to the reconstructive procedure, the patient reported he “did not think he would be able to live past this point in his life” and considered discontinuing treatment. However, since recovering from the procedure, he is satisfied with his treatment course and is “very grateful to the medical team”. The patient is now able to ambulate without assistance and without pain. Although still limited in his abilities, the patient says he currently enjoys watching television (specifically football games) and going for walks outside, both of which were insufferable prior to reconstruction and fusion.
Discussion
This case describes a complex treatment course of a VCF which was complicated by recurrent infections. It highlights the necessary precautions prior to KP procedure and emphasizes the risk of postoperative sepsis and osteomyelitis particularly in patients with comorbid conditions like diabetes. Postoperative infection following KP, while relatively rare, warrant additional preoperative screenings to mitigate risks. Between 2005 and 2010, a mere 0.11% of KP procedures resulted in postoperative infections (12). Nonetheless, data regarding post-operative infections with Corynebacterium species remains sparce (13).
Our patient’s history of diabetes and acute septicemia is a significant consideration in the decision-making process for KP. Similar cases have identified diabetic foot ulcers as the source of vertebral osteomyelitis. One case presented a 63-year-old male with a diabetic foot ulcer, infected with methicillin-resistant Staphylococcus aureus that progressed to calcaneal osteomyelitis and finally L4–L5 vertebral osteomyelitis, T9–L1 epidural abscess, and right psoas muscle abscess (10). The infection was suspected to have seeded hematogeneously from the foot to the spine. While it is unclear if our patient’s osteomyelitis stemmed from a foot ulcer, the potential for hematogenous spread underscores the necessity for cautious surgical consideration.
When it comes to managing osteomyelitis, literature reports both operative and nonoperative approaches. The decision to reoperate in this case also demonstrates treatment success via removal of hardware and subsequent posterior fusion. Yet, the report’s single-case basis limits the extension of this outcome as a universal treatment recommendation. Other cases have shown nonoperative management to be effective as well as described above (11).
Overall, there are key takeaways from this case that must be considered. This case emphasizes the importance of preoperative deliberation and adequate infection risk profiling prior to surgery for compression fracture. Lab values [C-reactive protein (CRP), ESR, and WBC] must be considered in addition to pertinent past medical history in situations that may increase infection risk. Furthermore, antibiotic-loaded bone cement should be considered in all KP cases when infection is suspected. While there is controversy regarding its efficacy and contribution to antibiotic resistance, its prophylactic use could prevent the potentially fatal complication as described in this case and its utility should not be undervalued (14). Taking these precautions may prevent postoperative complications in similar cases.
Additionally, although some cultures result in no growth, the intra-operative culture is still imperative to allow for targeted antibiotic therapy in the setting of post-operative infection. This precaution allows for swift identification of potential pathogens for effective and targeted antibiotic therapy, should it be necessary. Finally, this case serves as a call for further research on KP and its associated complications. One of the largest studies in literature analyzed 1,150 KPs over 7 years, but only recorded data for 30 days post operation (15). This case and others mentioned present with post-operative complications months after surgery. Studies with longer follow-up must be conducted to capture the true complication rate for KPs.
Conclusions
This case describes KP procedure in the setting of systemic infection with the debilitating complication of post-operative infection. While uncommon, this potentially fatal complication can be treated with operative or conservative management. Further research on KPs with extended follow-up duration must be conducted to determine true complication rate of KP and definitive treatment strategy guidelines for post-operative infection.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-24-31/rc
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-24-31/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-24-31/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 2014;29:2520-6. [Crossref] [PubMed]
- Hasserius R, Karlsson MK, Nilsson BE, et al. Prevalent vertebral deformities predict increased mortality and increased fracture rate in both men and women: a 10-year population-based study of 598 individuals from the Swedish cohort in the European Vertebral Osteoporosis Study. Osteoporos Int 2003;14:61-8. [Crossref] [PubMed]
- Suzuki N, Ogikubo O, Hansson T. The course of the acute vertebral body fragility fracture: its effect on pain, disability and quality of life during 12 months. Eur Spine J 2008;17:1380-90. [Crossref] [PubMed]
- Maravic M, Taupin P, Roux C. Hospital burden of vertebral fractures in France: influence of vertebroplasty. Osteoporos Int 2013;24:2001-6. [Crossref] [PubMed]
- Tsai YW, Hsiao FY, Wen YW, et al. Clinical outcomes of vertebroplasty or kyphoplasty for patients with vertebral compression fractures: a nationwide cohort study. J Am Med Dir Assoc 2013;14:41-7. [Crossref] [PubMed]
- Zampini JM, White AP, McGuire KJ. Comparison of 5766 vertebral compression fractures treated with or without kyphoplasty. Clin Orthop Relat Res 2010;468:1773-80. [Crossref] [PubMed]
- Edidin AA, Ong KL, Lau E, et al. Morbidity and Mortality After Vertebral Fractures: Comparison of Vertebral Augmentation and Nonoperative Management in the Medicare Population. Spine (Phila Pa 1976) 2015;40:1228-41. [Crossref] [PubMed]
- Shin JH, Ha KY, Kim KW, et al. Surgical treatment for delayed pyogenic spondylitis after percutaneous vertebroplasty and kyphoplasty. Report of 4 cases. J Neurosurg Spine 2008;9:265-72. [Crossref] [PubMed]
- Ivo R, Sobottke R, Seifert H, et al. Tuberculous spondylitis and paravertebral abscess formation after kyphoplasty: a case report. Spine (Phila Pa 1976) 2010;35:E559-63. [Crossref] [PubMed]
- Nicolosi N, Pratt C. Infectious Spondylodiscitis, Epidural Phlegmon, and Psoas Abscess Complicating Diabetic Foot Infection: A Case Report. J Foot Ankle Surg 2016;55:267-71. [Crossref] [PubMed]
- Wendling D, Runge M, Toussirot E, et al. Vertebral osteitis adjacent to kyphoplasty. Joint Bone Spine 2010;77:67-9. [Crossref] [PubMed]
- Goz V, Errico TJ, Weinreb JH, et al. Vertebroplasty and kyphoplasty: national outcomes and trends in utilization from 2005 through 2010. Spine J 2015;15:959-65. [Crossref] [PubMed]
- Kalt F, Schulthess B, Sidler F, et al. Corynebacterium Species Rarely Cause Orthopedic Infections. J Clin Microbiol 2018;56:e01200-18. [Crossref] [PubMed]
- Bistolfi A, Massazza G, Verné E, et al. Antibiotic-loaded cement in orthopedic surgery: a review. ISRN Orthop 2011;2011:290851. [Crossref] [PubMed]
- McArthur N, Kasperk C, Baier M, et al. 1150 kyphoplasties over 7 years: indications, techniques, and intraoperative complications. Orthopedics 2009;32:90. [Crossref] [PubMed]





