
Minimally invasive prone lateral retropleural or retroperitoneal antepsoas approach spinal surgery using the rotatable radiolucent Jackson table
Highlight box
Key findings
• Minimally invasive lateral retropleural and retroperitoneal antepsoas approach surgery can be safely performed in the rotated prone position using a regular rotatable radiolucent Jackson table.
What is known and what is new?
• Single position dual approach surgery is a minimally invasive surgical option that allows simultaneous lateral and posterior access to the vertebral column, enhancing surgical room efficiency.
• Compared to staged lateral and posterior flip surgery, minimally invasive prone lateral retropleural or antepsoas surgery is a feasible alternative for a wide range of disease etiologies throughout thoracolumbar and lumbar vertebral segments, especially in complex procedures requiring multiple flips.
What is the implication, and what should change now?
• Prone lateral spinal surgery can be considered for patients requiring combined lateral and posterior approach spinal surgery. Both lateral retropleural and retroperitoneal approaches can be integrated in combination with various posterior surgical procedures.
Introduction
Over the past two decades, retroperitoneal lumbar interbody fusion has emerged as a minimally invasive surgery (MIS) option for treating various spinal conditions (1-4). Advances in minimally invasive surgical instruments and retractors have significantly reduced muscle dissection and wound size during surgeries like transpsoas lateral lumbar interbody fusion (LLIF) and antepsoas oblique lumbar interbody fusion (OLIF). These techniques allow for increased disc height restoration, indirect neural element decompression, and large interbody graft placement, while preserving the posterior ligamentous complex (1,4,5).
The retropleural thoracic approach offers a minimally invasive alternative for addressing ventral thoracic pathologies, compared to more invasive methods like posterior laminectomy-based transpedicular, costotransversectomy, and lateral extracavitary approaches (6-8). It also reduces the need for one-lung ventilation and lowers pulmonary complication risks compared to the transpleural thoracic approach (9). This approach can be combined with the retroperitoneal approach at the thoracolumbar region using an MIS tubular retractor, without diaphragm takedown (6,10-12).
Recently, the single-position prone lateral transpsoas approach has been introduced as an MIS surgical option, offering simultaneous lateral and posterior access, and enhancing surgical room efficiency (13-16). For most spine surgeons, prone position is more familiar to performing posterior techniques like pedicle screw insertion, cement augmentation, decompression, and osteotomy (17,18). Additional advantages of doing a minimal invasive lateral approach in prone position include better lordosis restoration, a more posterior-oriented iliopsoas and lumbar plexus, and great saving the flip transition time (19-25). However, neuromonitoring like free-run or trigger electromyography (EMG) is needed when performing transpsoas approach due to the risk of lumbar plexus injury (1,4,26). In this study, we explore the feasibility of single-position minimally invasive prone lateral retropleural or retroperitoneal antepsoas approach surgery using a rotatable radiolucent Jackson table and present a consecutive series of patients with different etiologies undergoing this procedure and their surgical outcomes. We present this article in accordance with the STROBE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-24-71/rc).
Methods
Patients and data collection
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). After obtaining institutional review board approval from Chang Gung Medical Foundation (IRB No. 202400604B0), we retrospectively reviewed all adult patients who underwent simultaneous minimally invasive lateral and posterior approached surgery in the prone position for various etiologies at Chang Gung Memorial hospital from July 2021 to June 2023. Individual consent for this retrospective analysis was waived. Patients who received lateral approached surgeries in lateral decubitus positions, or patients who followed up for less than 1 year were excluded. We reviewed the medical charts to collect patient demographics, medical comorbidities, disease etiologies, and details of surgical procedures. Clinical outcomes, sagittal Cobb angles of lateral surgical level, postoperative complications, Visual Analogue Scale (VAS) and Oswestry Disability Index (ODI) before and after 1 year of the operation were also analyzed.
Preoperative positional preparation
Before surgery, it is imperative for the spine surgeon to communicate thoroughly with the anesthesiologist about the need to undertake a prone lateral surgery, in which the patient will be rotated 30–40 degrees for a certain duration of time intraoperatively. The position of the four-post frame on the radiolucent Jackson table needs to be adjusted for the lateral approach. The hip pad on the approach side should be positioned downward to the position of the upper thigh (Figure 1A). This will allow full exposure of the working space on the approach side from the ribs to the iliac crest when the patient is in the prone position (Figure 1B).

A Mizuho Jackson Modular Table System is used for all prone lateral surgeries. After general anesthesia, the patient is then turned to a prone position on the rotatable radiolucent Jackson table. Once the patient is in the prone position, two lateral positioners are placed on the contralateral side ribs and femoral trochanteric area as support after rotation and backstop during graft placement (Figure 1C). The position of the lateral positioner should avoid obstructing the intraoperative fluoroscopic view of the upper and lower instrumented vertebrae. For patients receiving spinopelvic fixation, the lateral positioner at the femoral trochanteric area should avoid blocking the tear drop under the obturator oblique view (27). Tape is then used to secure the thoracic and pelvic area of the patient, avoiding the sterilized area and surgical field. Both legs and arms are secured with straps and tape while the head is taped to the table with care, avoiding excessive tension of the tape to the head and pressure on the eyes. The head should remain within the head positioning cushions after the table has been rotated.
Once the patient is secured and the table has been elevated to a comfortable height for the surgeon, the patient is manually rotated 30–40 degrees away from the approach side (Figure 1D-1F). The rotation should be about 30 degrees for the thoracic or thoracolumbar spine, whereas the lower lumbar (L3–5) should be rotated to about 40 degrees for a better approach to the antepsoas corridor. The lateral and posterior surgical levels are then marked under true lateral and anteroposterior (AP) images using a fluoroscope C-arm, with the endplates and pedicles parallel on the lateral view and with the spinous process bisecting the pedicles on the AP view. For a lateral approach, the planned skin incision is marked anterior to the targeted vertebrae if approaching lower lumbar segments (Figure 1G). The skin incision mark is drawn slightly more posteriorly when approaching thoracic or thoracolumbar segments, centered over the closest rib to the targeted level which will be harvested later as autograft (Figure 1H). A 4–5 cm incision is usually sufficient for a 1- or 2-level discectomy. After marking the skin incision, the patient is then carefully sterilized and draped to expose the lateral surgical corridor and posterior exposure area with the C-arm machine fixed in the true AP view position (Figure 1I). True lateral view is obtained by rotating the C-arm over the patient (Figure 1J). The upper part of the C-arm at the top of the patient also needs to be covered with a sterile drape. The specific rotation of the C-arm in each direction can be marked at this time to ensure ease in returning to these true AP and lateral images (Figure 1K,1L) later.
Surgical techniques
Using L2–L5 lower lumbar segment OLIF as an example of the minimally invasive lateral approach surgery in prone position, it follows the same procedure in a lateral decubitus position (28) (Figure 2A). After incision of the skin and subcutaneous fascia, the external oblique, internal oblique, and transverse abdominis are successively separated by muscle splitting. The retroperitoneal fat and peritoneal content are retracted ventrally using finger dissection until the psoas muscle is palpated. Dissecting down and exposing the psoas is more challenging with the patient in the prone position due to the greater depth of the field compared to the lateral decubitus position where gravity aids in lowering the abdominal content. A long Kocher clamp with a gauze pusher can be used to strip the fascia on the psoas muscle and identify the targeted disc level along the anterior edge of the psoas muscle. The psoas muscle at the target disc level is slightly mobilized, enabling the antepsoas working corridor to about 2–3 cm. A Kirschner wire is inserted into the targeted disc at an angle approximately parallel to the ground, which equals an angle of 30–40 degrees obliquely to the coronal plane of the vertebral body (Figure 2B). The insertion point is confirmed using fluoroscopic AP & lateral view to be between the anterior half to anterior one-third of the disc level in lateral view. Serial dilators are inserted with fingers protecting the abdominal content from being trapped into the dilators. A table-mounted OLIF retractor is docked with the psoas muscle retracted dorsally. No muscle should protrude from underneath the retractor blades. Similar to the decubitus OLIF, a thorough discectomy is performed under direct vision and fluoroscopic guidance after annulotomy. Endplates are prepared under fluoroscope guidance using a Cobb elevator to avoid endplate injury. After serial trialing to the proper size of the cage, the OLIF interbody cage packed with a combination of allograft, artificial bone graft, or demineralized bone matrix (DBM) is inserted. The cage position is checked under fluoroscopic AP & lateral views. More complex procedures like mini-open corpectomy, anterior column realignment (ACR), or tumor excision can also be performed with a wider approach window in this position.
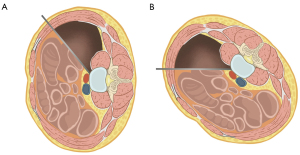
For a lateral approach to thoracic or thoracolumbar segments, circumferential subperiosteal dissection of the previously marked rib is performed after skin incision and fascial dissection. Right-side approach in the thoracic segments (T8–T11) was typically chosen to avoid mobilization of the aorta. For thoracolumbar (T11–L2) segments, the left-side approach was mostly utilized due to its potential connection to the left retroperitoneal antepsoas approach. The approach side may vary depending on the laterality of the lesion. The rib should be dissected further than the skin incision after undermining and mobilizing the subcutaneous fascia. A rib cutter and rongeur are used for the partial rib resection after freeing the rib from the subcostal neurovascular bundle and parietal pleura. The retropleural space is carefully developed between the plane of endothoracic fascia and parietal pleura as described by Uribe et al. (7). A generous retropleural blunt dissection above and below the targeted disc level under the adjacent ribs is performed to avoid incidental parietal pleura tear. The rib head near the targeted disc level is identified and blunt dissection is carried to the anterior vertebral body. A table-mounted OLIF retractor is docked to retract the lung and parietal pleura. Then lateral procedures like discectomy, mini-open corpectomy and interbody reconstruction and fusion can be performed similarly to the MIS lateral lumbar retroperitoneal approach.
Our typical workflow for minimally invasive prone lateral surgery usually begins with MIS lateral interbody fusion followed by percutaneous posterior pedicle screw fixation. If the surgery needs to begin with the posterior approach, the table is rotated back to the horizontal position after draping. Posterior-based surgical procedures like open/percutaneous pedicle screw insertion, decompression, osteotomy, cement augmentation, or endoscopic surgery can all be performed after simply rotating the table back to the true horizontal position as checked by the fluoroscope. For complex or revision surgery which typically requires multiple flips, like posterior to lateral to posterior approaches, simultaneous lateral and posterior manipulation in the prone position is also possible without the need to re-drape the patient. During MIS lateral approach surgery, the surgeon operates at the ventral side and can choose to stand or sit on a stool, depending on the surgeon’s ergonomic approach angle. After completing the surgery, both lateral and posterior wound closure are performed in a standard fashion. A drain is placed when performing complex surgeries. Significant pleural violations may necessitate the placement of a chest tube.
Statistical analysis
The clinical and radiographic outcomes of preoperative and postoperative sagittal Cobb angle of lateral surgical level (kyphosis was determined as minus value; whereas lordosis was determined as plus value), VAS and ODI were compared with paired-t test or Wilcoxon signed-rank test. The Shapiro-Wilk test was employed to assess whether the data followed a normal distribution. Subgroup analyses of both prone lateral retropleural and retroperitoneal approaches were performed. Statistical significance was defined as P value <0.05. The analyses were conducted using SPSS Version 25 (IBM Corporation, Armonk, NY, USA).
Results
Patient demographics and operative details
There is a consecutive series of 64 patients receiving minimally invasive prone lateral spinal surgeries within the study interval. All of the operations were performed by the same surgical team. The demographic data and operative details are listed in Table 1. There were 30 females and 34 males with a mean age of 61.8±12.8 years (range, 26–88 years). The American Society of Anesthesiologists (ASA) gradings were as follows: I, 3 (4.7%); II, 9 (14.1%); III, 50 (78.1%); and IV, 2 (3.1%). The spinal disease etiologies of all patients were 15 (23.4%) degenerations, 11 (17.2%) deformities, 25 (39.1%) infections, 9 (14.1%) traumas, and 4 (6.3%) tumors. Case examples in each etiology were presented as Figures 3-7.
Table 1
| Variables | Results |
|---|---|
| Age (years) | 61.8±12.8 [26–88] |
| Sex (F/M) | 30/34 |
| Body height (cm) | 161.5±9.3 |
| Body weight (kg) | 66.3±13.1 |
| BMI (kg/m2) | 25.3±4.3 |
| ASA grading | |
| I | 3 (4.7) |
| II | 9 (14.1) |
| III | 50 (78.1) |
| IV | 2 (3.1) |
| Disease etiology | |
| Degeneration | 15 (23.4) |
| Deformity | 11 (17.2) |
| Infection | 25 (39.1) |
| Trauma | 9 (14.1) |
| Tumor | 4 (6.3) |
| Operative time (minutes) | 314±148 [92–785] |
| Blood loss (mL) | 863±843 [50–4,600] |
| Length of surgical level | 4.1±2.0 [2–10] |
| Surgical level | |
| Lateral | T8–L5 |
| Posterior | T6–ilium |
| Revision surgery | 10 (15.6) |
| Lateral surgery for previously operated level | 9 (14.1) |
| Lateral surgery | |
| Retropleural approach | 25 |
| Retroperitoneal approach | 39 |
| Antepsoas | 36 |
| Transpsoas | 3 |
| Procedures | |
| Discectomy and fusion | 63 |
| 1-level | 26 |
| 2-level | 29 |
| 3-level | 7 |
| 4-level | 1 |
| Corpectomy | 26 |
| Lateral instrumentation | 8 |
| En bloc spondylectomy | 3 |
| Posterior surgery | |
| Pedicle screw instrumentation | 64 |
| Open | 20 |
| Percutaneous | 44 |
| Cement augmentation | 20 |
| Other posterior procedures* | 20 |
Data are presented as mean ± standard deviation [range], n or n (%). *, including posterior decompression, Ponte osteotomy, screw removal, debridement, and spinal endoscope. F, female; M, male; BMI, body mass index; ASA, American Society of Anesthesiologists.





The mean operation time was 314±148 minutes (range, 92–785 minutes) and the mean blood loss was 863±843 mL (range, 50–4,600 mL). The mean length of the surgical level was 4.1±2.0 (range, 2–10). The lateral surgical level ranged from T8 to L5 and the posterior surgical level ranged from T6 to ilium. There were 10 (15.6%) patients receiving revision surgery and 9 (14.1%) of them received lateral surgery for the previously operated levels.
Of the lateral approaches, there were 25 thoracic or thoracolumbar retropleural (2 from right side) and 39 lumbar retroperitoneal (1 from right side) approaches. The spinal disease etiologies of the 25 retropleural approaches were 6 (24%) deformities, 10 (40%) infections, 5 (20%) traumas, and 4 (16%) tumors, while the etiologies of the 39 retroperitoneal approaches were 15 (38%) degenerations, 5 (13%) deformities, 15 (38%) infections, and 4 (10%) traumas. Among the 39 retroperitoneal approaches, 36 underwent antepsoas and 3 underwent transpsoas approaches (1 from right side and 2 from left side). All of the 3 patients receiving transpsoas approach were due to drainage of psoas muscle abscess and used the transpsoas window for lateral discectomy and debridement.
For minimally invasive lateral procedures, there were 63 patients received lateral discectomy and fusion (1-level: 26; 2-level: 29; 3-level: 7; 4-level: 1), 26 received mini-open lateral corpectomies (1-level: 22; 2-level: 4), 8 had lateral instrumentation. There were 3 patients underwent single level en bloc spondylectomy using the prone lateral combined approach (2 patients at L1, 1 patient at L2).
For posterior pedicle screw instrumentation, there were 20 patients used open method and 44 patients used percutaneous method. There were 20 patients received additional cement augmentation and 20 patients received other posterior surgical procedures like posterior decompression, Ponte osteotomy, screw removal, debridement, and spinal endoscope.
Clinical and radiographic outcomes
The clinical and radiographic outcomes for all patients and different etiologies treated with prone lateral retropleural and retroperitoneal approaches were listed in Tables 2,3. There were significant improvements in sagittal Cobb angles of lateral surgical level, ODI and VAS between before operation and 1 year postoperatively (all P<0.05) except for the tumor patients. For prone lateral retropleural group, the sagittal Cobb angle of lateral surgical level improved from −20.8°±18.0° to 7.7°±14.0° with a mean difference of 28.5°±15.7° (P<0.001). The ODI improved from 40.8±5.2 to 18.9±9.3 and the VAS also improved from 7.7±0.9 to 3.3±1.7 (both P value <0.001). For prone lateral retroperitoneal group, the sagittal Cobb angle of lateral surgical level improved from −3.6°±17.2° to 20.9°±9.6° with a mean difference of 17.3°±18.2°, the ODI improved from 36.8±8.4 to 13.7±10.2 and the VAS also improved from 7.4±1.1 to 2.3±1.8 (all P<0.001).
Table 2
| Variables | All (N=25) | Deformity (N=6) | Infection (N=10) | Trauma (N=5) | Tumor (N=4) |
|---|---|---|---|---|---|
| Length of surgical level | 5.3±1.9 [3–10] | 6.3±2.3 [4–10] | 5.7±2.3 [3–8] | 3.4±0.9 [3–5] | 5.0±0.0 [5] |
| OP time (minutes) | 372±156 | 430±163 | 321±126 | 268±88 | 542±140 |
| Blood loss (mL) | 1,104±821 [200–3,000] |
1,217±816 [200–2,200] |
1,155±1,065 [200–3,000] |
710±338 [300–1,200] |
1,300±623 [750–2,000] |
| Surgical level | |||||
| Lateral | T8–L5 | T11–L5 | T8–L4 | T11–L2 | T12–L3 |
| Posterior | T6–ilium | T10–ilium | T6–L4 | T10–L2 | T11–L4 |
| Sagittal Cobb angle of lateral surgical level | |||||
| PreOP | −20.8°±18.0° | −26.9°±26.3° | −19.9°±11.3° | −30.1°±16.4° | −2.5°±6.4° |
| PostOP | 7.7°±14.0° | 13.8°±20.8° | 1.3°±8.9° | 5.6°±6.6° | 16.9°±15.0° |
| Difference | 28.5°±15.7° | 40.7°±10.5° | 21.2°±14.8° | 35.7°±15.7° | 19.4°±11.2° |
| P value | <0.001 | 0.03 | 0.005 | 0.043 | 0.07 |
| ODI | |||||
| PreOP | 40.8±5.2 | 40.3±2.3 | 42.9±1.8 | 43.6±2.5 | 32.8±8.6 |
| PostOP | 18.9±9.3 | 16.3±9.9 | 21.1±8.7 | 14.4±11.7 | 22.8±5.3 |
| P value | <0.001 | 0.03 | 0.005 | 0.043 | 0.14 |
| VAS | |||||
| PreOP | 7.7±0.9 | 7.5±0.5 | 8.0±0.7 | 8.2±0.8 | 6.8±1.5 |
| PostOP | 3.3±1.7 | 3.0±1.1 | 3.8±1.5 | 1.8±2.0 | 3.3±2.1 |
| P value | <0.001 | 0.03 | 0.005 | 0.043 | 0.20 |
Data are presented as mean ± standard deviation or [range]. Paired-t test or Wilcoxon signed-rank test was used to obtain the P value. OP, operative; ODI, Oswestry Disability Index; VAS, Visual Analogue Scale.
Table 3
| Variables | All (N=39) | Degeneration (N=15) | Deformity (N=5) | Infection (N=15) | Trauma (N=4) |
|---|---|---|---|---|---|
| Length of surgical level | 3.5±1.8 [2–10] | 2.5±0.6 [2–4] | 5.8±3.3 [3–10] | 3.5±1.4 [2–6] | 4.3±1.7 [2–6] |
| OP time (minutes) | 276±131 | 208±85 | 473±201 | 267±75 | 319±121 |
| Blood loss (mL) | 709±830 [50–4,600] |
253±168 [50–650] |
1,490±1,761 [350–4,600] |
720±419 [100–1,400] |
1,400±979 [200–2,200] |
| Surgical level | |||||
| Lateral | L1–L5 | L3–L5 | L2–L5 | L1–L5 | L2–L5 |
| Posterior | T10–ilium | L2–L5 | T10–ilium | T12–ilium | T12–ilium |
| Sagittal Cobb angle of lateral surgical level | |||||
| PreOP | −3.6°±17.2° | 12.1°±8.0° | −18.2°±31.5° | 5.4°±12.4° | −7.3°±8.1° |
| PostOP | 20.9°±9.6° | 20.3°±8.3° | 25.5°±10.1° | 17.7°±9.7° | 29.6°±8.5° |
| Difference | 17.3°±18.2° | 8.2°±5.0° | 43.8°±29.3° | 12.3°±12.2° | 36.9°±2.0° |
| P value | <0.001 | 0.001 | 0.043 | 0.003 | 0.07 |
| ODI | |||||
| PreOP | 36.8±8.4 | 27.6±4.9 | 39.8±4.0 | 43.3±2.4 | 43.5±4.4 |
| PostOP | 13.7±10.2 | 6.5±3.4 | 10.4±4.5 | 21.1±11.1 | 17.5±9.6 |
| P value | <0.001 | 0.001 | 0.043 | 0.001 | 0.07 |
| VAS | |||||
| PreOP | 7.4±1.1 | 6.4±0.8 | 7.6±0.9 | 8.1±0.8 | 8.3±1.0 |
| PostOP | 2.3±1.8 | 0.9±1.2 | 2.2±1.3 | 3.5±1.6 | 3.0±1.2 |
| P value | <0.001 | 0.001 | 0.043 | 0.001 | 0.07 |
Data are presented as mean ± standard deviation or [range]. Paired-t test or Wilcoxon signed-rank test was used to obtain the P value. OP, operative; ODI, Oswestry Disability Index; VAS, Visual Analogue Scale.
The postoperative complications followed by prone lateral surgery were listed in Table 4. The approach-related complication rate is 9% (6/64). Among the approach-related complications, there are 5 (13%) out of 39 retroperitoneal approach patients developed transient left thigh pain/weakness. All of them recovered within 3 months of surgery. There was no peritoneal organ injury during the retroperitoneal approach. One multiple trauma patient with L1 compression fracture and L3 comminuted burst fracture received L1 vertebroplasty, prone lateral antepsoas L3 corpectomy and L2–4 percutaneous cemented instrumentation developed left lower abdominal wall abscess formation and hernia and received laparoscopic debridement and repair of ventral hernia at postoperative 5 months (Figure 6). For the deformity correction-related complications, one patient developed recurrent dislocation of previous implanted total hip arthroplasty and received subsequent revision total hip arthroplasty surgeries. There were 3 junctional failures with 2 proximal junctional kyphosis and 1 distal junctional kyphosis requiring revision surgeries.
Table 4
| Complications | No. of patients |
|---|---|
| Approach-related | |
| Transient left thigh pain/weakness | 5 |
| Abdominal wall abscess and hernia | 1 |
| Deformity correction-related | |
| Recurrent dislocation of total hip arthroplasty | 1 |
| Proximal junctional kyphosis | 2 |
| Distal junctional kyphosis | 1 |
Discussion
Recent advancements in spinal surgery have emphasized the efficacy of single-position dual approaches in treating degenerative lumbar disease (13,17). This method, aligned with MIS principles, offers multi-dimensional benefits. It reduces the need for patient repositioning, enhances operating room efficiency, and facilitates simultaneous anterior and posterior column manipulation (20). Compared to staged flip surgeries requiring patient repositioning, a multi-center study utilizing the Lateral decubitus position for simultaneous LLIF or anterior lumbar interbody fusion (ALIF) and percutaneous pedicle screw instrumentation reported reduced blood loss, shorter hospital stays, and decreased postoperative ileus, while maintaining similar incidences of wound complications, vascular injury, neurological complications, or venous thrombotic events (29). A meta-analysis further indicates that single-position surgeries, either in decubitus or prone positions, shorten operative times and hospital stays without compromising complication rates or radiographic outcomes (30). Compared to the posterior approach surgery in decubitus position, the prone position is more familiar and comfortable for most spinal surgeons, allowing for the execution of various techniques in complex scenarios (26). With its benefits in posterior-based surgical techniques, performing lateral surgery in the prone position has proven to be a safe and efficient alternative for revision lumbar fusion, compared to staged flip surgeries (20,31). This approach is also viable for complex cases requiring posterior high-grade three-column osteotomy, with prone transpsoas lumbar corpectomy enabling simultaneous posterior and lateral access (32,33).
Emerging literature has shed light on other advantages of lateral surgery in the prone position. A meta-analysis demonstrated that the restoration of segmental lordosis in lateral surgery is significantly better in the prone position than in the decubitus position (30). Serial anatomical studies indicate that in the prone position with hip extension, the psoas muscle is least anteriorly elongated, resembling its positioning in supine magnetic resonance imaging (MRI) (19,21,22). The psoas shows substantial lateral mobility and posterior retraction at L5 in this position (19). Another cadaveric study also revealed that the femoral nerve of the lumbar plexus is more posteriorly located at the L4–L5 disc space in the prone position compared to the lateral decubitus position, potentially lowering the risk of femoral nerve injury in prone transpsoas approach surgeries (21). In this position, major vessels like the inferior vena cava (IVC) and right iliac vein experience significant anterior mobility, particularly at higher levels. Although the retroperitoneal organs shift away from the operative field in the prone position, they remain close enough to be at risk of injury (34). The overall low rate of retroperitoneal or peritoneal injury makes it difficult to demonstrate a lower risk in the prone position (35).
In this study, we detail the application of a minimally invasive prone lateral combined approach for spinal surgery on 64 patients using a rotatable radiolucent Jackson table. By rotating the prone patient 30–40 degrees, we could perform various MIS lateral techniques at thoracolumbar segments from T8 to L5, such as discectomy, fusion, mini-open corpectomy, lateral instrumentation, and even combined approaches for single-level en bloc spondylectomy in the lumbar spine. While some surgeons have recommended rotating the operating table by 10–30 degrees to improve ergonomics and enhance lateral access visualization during prone transpsoas surgery (13,16,25), it is crucial to recognize that the trajectory for the prone antepsoas approach differs from that of the prone transpsoas approach. A 30–40-degree rotation has aligned the surgeon’s horizontal vision line with the oblique trajectory utilized for OLIF in the decubitus position (Figure 2). Surgeons must carefully dissect the retroperitoneal space to avoid ventral incursion that could jeopardize peritoneal organs. The psoas muscle, transverse process tip, and iliac crest could serve as the landmark when approaching to the lower lumbar spine. Utilizing a table-mounted retractor enables retraction of the psoas muscle and peritoneal organs, thereby revealing the antepsoas OLIF corridor. When performing the OLIF orthogonal maneuver in this rotated prone position, it is imperative for surgeons to know that an oblique downward angle of 30–40 degrees corresponds with the true lateral direction. Despite the differing orientation from the decubitus position, surgeons were able to adapt with the help of intraoperative fluoroscopic guidance and performed various lateral techniques with adjusted directions.
Similar to the prone retroperitoneal antepsoas approach, the minimally invasive prone retropleural approach can be performed at the thoracolumbar region without diaphragm takedown using the rotatable radiolucent table. This technique streamlines complex cases by facilitating simultaneous lateral and posterior approaches, therefore negating the need for multiple flips and re-drapes. With this technique, a three-column osteotomy can be transitioned to an ACR (36) with low-grade posterior osteotomy in adult spinal deformity correction without the need for re-draping (Figure 4). This method is also applicable to treating infective spondylodiskitis through a minimally invasive lateral approach, complemented by percutaneous posterior instrumentation (Figure 5). For single-level lumbar en bloc spondylectomy, it allows the simultaneous execution of lateral and posterior procedures, enabling tumor and anterior vertebral body removal without excessive retraction of the lumbar roots (Figure 7). With the patient in prone position, various MIS posterior procedures like percutaneous vertebroplasty or spinal endoscope could also be done easily in conjunction with the lateral surgery. However, this approach is not without its trade-offs. A combined lateral and posterior approach for complex spinal deformities often leads to longer, single-stage surgeries, which carry increased risks of anesthesia-related complications and pressure sores. To mitigate this, we have incorporated the use of thick silicone pads at the four-post frame and two lateral positioners on the contralateral side. Additionally, while the 30–40 degrees rotated prone position demands fluoroscopic guidance for surgical orientation—less intuitive than the lateral decubitus position—it may present more challenges for morbidly obese patients. For obese patient with high BMI, longer instruments and retractor blades must be used. The retractor could be placed to the ideal place after adjustments under both direct vision and fluoroscopic guidance. The authors used the headlight to facilitate vision in the minimal invasive wound. In our series, the most corpulent patient underwent successful surgery has a BMI of 36.4. Additional care must be taken to adjust surgical incisions for obese patients combined with a high iliac crest when accessing lower lumbar segments. The surgical incision should be more anterior to avoid being hindered by the high iliac crest. Larger rotational angle (>40 degrees) is another strategy to overcome a high iliac crest. However, the surgeons should be aware of the rotatory angle and surgical orientation while doing the discectomy and cage insertion under fluoroscopic guidance. According to literatures, 34% of the patients developed transient femoral neuropraxia after prone LLIF and the strength of the left hip flexor muscle decreased by 22.6% followed by decubitus OLIF (37,38). In our study, 13% of the patient developed transient left thigh pain/weakness and all of them resolved within 3 months postoperatively.
This study has certain limitations. Primarily, it is a retrospective case series with only short-term follow-up and lacks a control group for benchmarking against standard two-staged surgeries in both decubitus and prone positions. Nonetheless, it is pioneering in detailing the minimally invasive retroperitoneal antepsoas and retropleural approaches in the rotated prone position. The early clinical outcomes and a low approach-related complication rate suggest its viability for a range of pathologies, including degeneration, deformity, infection, trauma, and tumors. A navigation system was not employed, which could have reduced fluoroscopic radiation exposure to the medical staff and potentially increased surgical efficiency. Future research should include a larger, prospective comparative cohort study with extended follow-up to refine the treatment algorithm and identify any limitations of this technique.
Conclusions
Our study demonstrates the feasibility of minimally invasive prone lateral spinal surgery with the good clinical and radiographic outcomes while maintaining acceptable complication rates. Both lateral retropleural and retroperitoneal antepsoas approaches, in combination with various posterior procedures, can be effectively performed in the prone position using a rotatable radiolucent Jackson table. This method presents a promising option for patients requiring complex spinal surgeries. To gain a deeper understanding of the safety profile and long-term outcomes, future research involving larger patient cohorts and extended follow-up periods is essential.
Acknowledgments
This research has been presented at the Global Spine Congress 2024 as an oral presentation.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-24-71/rc
Data Sharing Statement: Available at https://jss.amegroups.com/article/view/10.21037/jss-24-71/dss
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-24-71/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-24-71/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Chang Gung Medical Foundation (No. 202400604B0) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- White MD, Uribe JS. Transpsoas Approaches to the Lumbar Spine: Lateral and Prone. Neurosurg Clin N Am 2023;34:609-17. [Crossref] [PubMed]
- DiGiorgio AM, Edwards CS, Virk MS, et al. Stereotactic navigation for the prepsoas oblique lateral lumbar interbody fusion: technical note and case series. Neurosurg Focus 2017;43:E14. [Crossref] [PubMed]
- Mehren C, Mayer HM, Zandanell C, et al. The Oblique Anterolateral Approach to the Lumbar Spine Provides Access to the Lumbar Spine With Few Early Complications. Clin Orthop Relat Res 2016;474:2020-7. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2-18. [Crossref] [PubMed]
- Hung SF, Liao JC, Tsai TT, et al. Comparison of outcomes between indirect decompression of oblique lumbar interbody fusion and MIS-TLIF in one single-level lumbar spondylosis. Sci Rep 2021;11:12783. [Crossref] [PubMed]
- Krafft PR, Noureldine MHA, Greenberg MS, et al. Minimally Invasive Lateral Retropleural Approach to the Thoracic Spine for Salvage of a Subsided Expandable Interbody Cage. World Neurosurg 2020;135:58-62. [Crossref] [PubMed]
- Uribe JS, Dakwar E, Cardona RF, et al. Minimally invasive lateral retropleural thoracolumbar approach: cadaveric feasibility study and report of 4 clinical cases. Neurosurgery 2011;68:32-9; discussion 39. [Crossref] [PubMed]
- Angevin PD, McCormick PC. Retropleural thoracotomy. Technical note. Neurosurg Focus 2001;10:ecp1. [Crossref] [PubMed]
- Soda C, Faccioli F, Marchesini N, et al. Trans-thoracic versus retropleural approach for symptomatic thoracic disc herniations: comparative analysis of 94 consecutive cases. Br J Neurosurg 2021;35:195-202. [Crossref] [PubMed]
- Noureldine MHA, Pressman E, Krafft PR, et al. Minimally Invasive Lateral Retropleural and Retroperitoneal Approaches in Patients with Thoracic and Lumbar Osteomyelitis: Description of the Techniques and a Series of 14 Patients. World Neurosurg 2020;139:e166-81. [Crossref] [PubMed]
- Christiansen PA, Huang S, Smith JS, et al. Mini-open lateral retropleural/retroperitoneal approaches for thoracic and thoracolumbar junction anterior column pathologies. Neurosurg Focus 2020;49:E13. [Crossref] [PubMed]
- Yen CP, Uribe JS. Mini-open Lateral Retropleural Approach for Symptomatic Thoracic Disk Herniations. Clin Spine Surg 2018;31:14-21. [Crossref] [PubMed]
- Martirosyan NL, Uribe JS, Randolph BM, et al. Prone Lateral Lumbar Interbody Fusion: Case Report and Technical Note. World Neurosurg 2020;144:170-7. [Crossref] [PubMed]
- Lamartina C, Berjano P. Prone single-position extreme lateral interbody fusion (Pro-XLIF): preliminary results. Eur Spine J 2020;29:6-13. [Crossref] [PubMed]
- Godzik J, Ohiorhenuan IE, Xu DS, et al. Single-position prone lateral approach: cadaveric feasibility study and early clinical experience. Neurosurg Focus 2020;49:E15. [Crossref] [PubMed]
- Pimenta L, Taylor WR, Stone LE, et al. Prone Transpsoas Technique for Simultaneous Single-Position Access to the Anterior and Posterior Lumbar Spine. Oper Neurosurg (Hagerstown) 2020;20:E5-E12. [Crossref] [PubMed]
- Farber SH, Naeem K, Bhargava M, et al. Single-position prone lateral transpsoas approach: early experience and outcomes. J Neurosurg Spine 2022;36:358-65. [Crossref] [PubMed]
- Alan N, Kanter JJ, Puccio L, et al. Transitioning from lateral to the prone transpsoas approach: flatten the learning curve by knowing the nuances. Neurosurg Focus Video 2022;7:V8. [Crossref] [PubMed]
- Munim MA, Nolte MT, Federico VP, et al. The Effect of Intraoperative Prone Position on Psoas Morphology and Great Vessel Anatomy: Consequences for Prone Lateral Approach to the Lumbar Spine. World Neurosurg 2024;181:e578-88. [Crossref] [PubMed]
- Buckland AJ, Proctor DJ, Thomas JA, et al. Single-Position Prone Lateral Lumbar Interbody Fusion Increases Operative Efficiency and Maintains Safety in Revision Lumbar Spinal Fusion. Spine (Phila Pa 1976) 2024;49:E19-24. [Crossref] [PubMed]
- Alluri R, Clark N, Sheha E, et al. Location of the Femoral Nerve in the Lateral Decubitus Versus Prone Position. Global Spine J 2023;13:1765-70. [Crossref] [PubMed]
- Gandhi SV, Dugan R, Farber SH, et al. Anatomical positional changes in the lateral lumbar interbody fusion. Eur Spine J 2022;31:2220-6. [Crossref] [PubMed]
- Courville E, Ditty BJ, Maulucci CM, et al. Effects of thigh extension on the position of the femoral nerve: application to prone lateral transpsoas approaches to the lumbar spine. Neurosurg Rev 2022;45:2441-7. [Crossref] [PubMed]
- Walker CT, Farber SH, Gandhi S, et al. Single-Position Prone Lateral Interbody Fusion Improves Segmental Lordosis in Lumbar Spondylolisthesis. World Neurosurg 2021;151:e786-92. [Crossref] [PubMed]
- Pimenta L, Pokorny G, Amaral R, et al. Single-Position Prone Transpsoas Lateral Interbody Fusion Including L4L5: Early Postoperative Outcomes. World Neurosurg 2021;149:e664-8. [Crossref] [PubMed]
- Patel HM, Fasani-Feldberg G, Patel H. Prone position lateral interbody fusion-a narrative review. J Spine Surg 2023;9:331-41. [Crossref] [PubMed]
- Yilmaz E, Abdul-Jabbar A, Tawfik T, et al. S2 Alar-Iliac Screw Insertion: Technical Note with Pictorial Guide. World Neurosurg 2018;113:e296-301. [Crossref] [PubMed]
- Woods KR, Billys JB, Hynes RA. Technical description of oblique lateral interbody fusion at L1-L5 (OLIF25) and at L5-S1 (OLIF51) and evaluation of complication and fusion rates. Spine J 2017;17:545-53. [Crossref] [PubMed]
- Buckland AJ, Ashayeri K, Leon C, et al. Single position circumferential fusion improves operative efficiency, reduces complications and length of stay compared with traditional circumferential fusion. Spine J 2021;21:810-20. [Crossref] [PubMed]
- Mills ES, Treloar J, Idowu O, et al. Single position lumbar fusion: a systematic review and meta-analysis. Spine J 2022;22:429-43. [Crossref] [PubMed]
- Soliman MAR, Diaz-Aguilar L, Kuo CC, et al. Complications of the Prone Transpsoas Lateral Lumbar Interbody Fusion for Degenerative Lumbar Spine Disease: A Multicenter Study. Neurosurgery 2023;93:1106-11. [Crossref] [PubMed]
- Stone LE, Diaz-Aguilar LD, Santiago-Dieppa DR, et al. Prone-lateral access to the lumbar spine: single-level corpectomy with approach discussion. Neurosurg Focus Video 2022;7:V9. [Crossref] [PubMed]
- Gandhi SD, Liu DS, Sheha ED, et al. Prone transpsoas lumbar corpectomy: simultaneous posterior and lateral lumbar access for difficult clinical scenarios. J Neurosurg Spine 2021;35:284-91. [Crossref] [PubMed]
- Dodo Y, Okano I, Kelly NA, et al. The anatomical positioning change of retroperitoneal organs in prone and lateral position: an assessment for single-prone position lateral lumbar surgery. Eur Spine J 2023;32:2003-11. [Crossref] [PubMed]
- Soliman MAR, Aguirre AO, Ruggiero N, et al. Comparison of prone transpsoas lateral lumbar interbody fusion and transforaminal lumbar interbody fusion for degenerative lumbar spine disease: A retrospective radiographic propensity score-matched analysis. Clin Neurol Neurosurg 2022;213:107105. [Crossref] [PubMed]
- Uribe JS, Schwab F, Mundis GM, et al. The comprehensive anatomical spinal osteotomy and anterior column realignment classification. J Neurosurg Spine 2018;29:565-75. [Crossref] [PubMed]
- Morgan CD, Katsevman GA, Godzik J, et al. Outpatient outcomes of patients with femoral nerve neurapraxia after prone lateral lumbar interbody fusion at L4-5. J Neurosurg Spine 2022;37:92-5. [Crossref] [PubMed]
- Lee S, Kim AR, Bang WS, et al. Psoas weakness following oblique lateral interbody fusion surgery: a prospective observational study with an isokinetic dynamometer. Spine J 2022;22:1990-9. [Crossref] [PubMed]


