
Microscopically-assisted Uninstrumented Surgical Tumor Decompression as an alternative to open surgery for symptomatic metastatic epidural spinal cord compression
Highlight box
Key findings
• Compared to standard open decompression with instrumented fusion for metastatic epidural spinal cord compression (MESCC), our proposed Microscopically-Assisted Uninstrumented Surgical Tumor Decompression (MUST-D) technique had shorter operating time, anesthesia duration, length of hospital stay, and time to ambulation.
• Despite no difference in mortality between groups, patients undergoing MUST-D surgery demonstrated significantly fewer complications, a shorter time to radiation therapy, improved 30-day ambulation, and no evidence of focal long-term instability.
What is known and what is new?
• The proposed MUST-D approach is a novel minimally invasive surgery (MIS) technique utilizing single-screw anchored vertebral augmentation without instrumentation for MESCC decompression.
• Direct surgical decompression with adjuvant radiotherapy has been previously shown to be superior to radiotherapy alone in improving ambulation and pain control for patients with MESCC.
What is the implication, and what should change now?
• Although 1-year mortality was not correlated with the surgical approach, the survival time was significantly longer with the MUST-D approach. The presence of spinal metastases does not usually correlate with survival, but in this case, the MIS approach did correlate with longer survival times and should be considered equivalent or superior to an open approach for survival benefit.
• The MUST-D surgical technique should be considered as first-line treatment for symptomatic focal MESCC to minimize complications, improve perioperative outcomes, and expedite adjuvant radiotherapy, especially for patients who may have been a poor candidate for a larger open approach.
Introduction
The spinal column is the third most common site of metastasis following the lung and liver (1,2). Spinal metastases affect 30–90% of all cancer patients, with approximately 10% of these patients experiencing symptomatic spinal cord compression (3). Furthermore, 94–98% of spinal cord compression cases display evidence of epidural and/or vertebral involvement (3). Symptomatic patients often present with severe back or neck pain and, to a lesser extent, radicular pain, extremity weakness, sensory disturbances, and/or bowel and bladder dysfunction (4,5).
The indications for surgical interventions for metastatic epidural spinal cord compression (MESCC) have evolved over the last 75 years with laminectomy procedures as the initial standard of care operation. However, these procedures often did not adequately decompress the primary tumor and further destabilized the spine. This technique was abandoned due to the worse outcomes compared to radiotherapy alone (6). The pivotal study by Patchell et al. demonstrated the superiority of direct surgical decompression and radiotherapy over radiotherapy alone for patients with MESCC in improving ambulation and pain control (7). This redirected a generation of oncologists and surgeons to perform instrumented vertebrectomies instead of laminectomy procedures alone (8).
The current goals for MESCC treatment are largely palliative, aiming to sustain or improve quality of life by preserving neurologic function, mechanical spinal stability, and pain reduction (9). Concerns with treatment include subsequent deformity from spinal instability, progression of neurological deficits, and complications from surgical, radiological, or oncological treatment. Active comorbidities further complicate treatment decisions, including the risk of blood clots from a baseline prothrombotic state, infection due to an immunosuppressive state from treatment regimens, and impairment in wound healing, creating higher surgical risk and complication rates (10,11). Thus, many patients are deemed non-operative candidates for open surgery due to age, comorbidities, metastatic burden, or limited life expectancy.
Open spine surgery for metastatic disease can have high rates of wound complications, often attributed to the reduction of circulation from midline incision or impaired healing after administration of radiation and chemotherapy (12,13). Therefore, postoperative cancer treatment is often delayed to reduce the risk of infection and dehiscence at the expense of prolonging immobilization and completion of adjuvant treatments (14). Delayed radiotherapy following surgery has been shown to increase the risk of local tumor progression with poorer local control and overall survival rates (15). In addition, postoperative ambulation function has been reported to be significantly associated with survival (16). When the primary treatment goal for MESCC is largely palliative, improving intraoperative variables, complication rates, and postoperative ambulation through less invasive surgical approaches may hold further importance in improving a patient’s remaining quality of life.
Minimally invasive surgical (MIS) techniques expand the pool of potential operative candidates for cancer patients with limited life expectancy by reducing perioperative morbidity and accelerating recovery (17-19). Today, the guiding principle in selecting a surgical approach for symptomatic MESCC is both to obtain adequate decompression of neural elements and provide stabilization to the spine while minimizing the risks of complications (20). MIS treatments have been shown to have smaller incisions, preserve anatomy, reduce iatrogenic tissue trauma, and decrease postoperative pain (21). However, few papers to date have been published describing MIS techniques for tumor decompression without instrumentation (19,22). Weller et al. demonstrated the utilization of posterolateral decompression without stabilization in patients with limited life expectancies and showed improvement in neurological outcomes in all eight patients (23). Similarly, Deutsch et al. later presented a posterolateral vertebrectomy and decompression without stabilization using a MIS approach in a case series of eight patients with no neurological deterioration at 1-year (22). Despite these favorable outcomes, the current literature is limited to small case series with no larger study to corroborate these findings.
Herein, we describe the Microscopically-Assisted Uninstrumented Spinal Tumor Decompression (MUST-D) technique for tumor decompression and treatment of symptomatic MESCC in comparison to the traditional open surgical approach (22). The MUST-D approach incorporates a tubular retractor with a posterior oblique approach, splitting the paraspinal muscles instead of retracting the muscles as performed in conventional midline open approaches. The MUST-D technique also avoids pedicle screw instrumentation, using only direct vertebral augmentation (VA) using cement and a single screw anchoring to stabilize the vertebral cavity. This technique avoids delays in adjuvant treatment with the small incision positioned outside the usual linear accelerator (LINAC) radiation field.
The purpose of this study is to compare the surgical outcomes of patients undergoing MUST-D or standard open instrumented approach for MESCC treatment. Key outcomes include 1-year mortality and survival, time to death, time to adjuvant radiation therapy (RTx), mobility, and degree of postoperative spinal instability. Secondary outcomes include baseline demographics, intraoperative and perioperative variables, and complication rates. We present this article in accordance with the STROBE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-24-135/rc).
Methods
This is a retrospective cohort study of patients undergoing MESCC decompression with the proposed MUST-D technique or standard open instrumented fusion at a single, multi-surgeon institution from November 2006 to June 2016. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was determined to be exempt from IRB oversight initially by the Icahn School of Medicine at Mount Sinai until expiration, and then by Advarra for the remainder of the study period. Individual consent for this retrospective analysis was waived.
MUST-D surgery was defined as MIS single-level partial vertebrectomy and cement augmentation with single screw anchoring. The MUST-D procedures were performed by the primary surgeon and open procedures by other surgeons at the same institution. These patients were identified through the Mount Sinai Data Warehouse. Demographic and clinical data were abstracted from patient charts. Identifiable markers were then removed prior to data input into password-protected databases. The final key was retained in a password-protected file separate from the primary data file.
Inclusion criteria for the study included: (I) symptoms indicating spinal instability or neural compression from apparent spinal metastasis or metastases. These include myelopathy, radiculopathy, or pathological fractures with radiographic signs of instability or deformity that causes pain; (II) radiographic evidence of MESCC, limited to no more than one full contiguous vertebral levels or involvement; (III) surgical confirmation of metastatic disease verified by the Department of Pathology at Mount Sinai Hospital. Patients were excluded if they lacked complete follow-up, such as post-discharge mortality, functional status, or neurological outcome. Patients with prior spinal surgery at the index level were also excluded, as were patients who required transthoracic vertebrectomy, or only underwent laminectomy, as the decompressive part of their treatment.
Data collection
Collected patient variables included baseline demographics, tumor histology, Spine Instability Neoplastic Score (SINS), Hauser Ambulation Index (HAI) score, and Cobb angle (24,25). The HAI assesses ambulation, functional status, and quality of life after symptomatic spinal metastatic disease treatment. The HAI evaluates mobility of patients regarding the time and degree of assistance on a 0 to 9 scale (Figure 1). A score of 0 indicates a fully active, asymptomatic independent ambulator, while a score of 9 indicates someone who is immobile and bedridden. The Cobb angle was measured on sagittal magnetic resonance imaging (MRI) and computed tomography (CT) as the angle resulting from the intersection of two perpendicular lines emanating from parallel lines constructed from the superior border of the superiorly affected vertebrae and the inferior border of the inferiorly affected vertebrae. The evaluation was based on pre-operative imaging within 30 days of surgery and post-operative imaging at least 30 days after surgery. Operative data included skin-to-closure surgical time, anesthesia duration, estimated blood loss (EBL), length of hospital stay, and level of operation. Outcome data included time to ambulation, time to RTx, 30-day postoperative complications, local recurrence, mortality, survival, and time to death.

Surgical approach
MUST-D surgery
A 1.7–2 cm incision was made 5–9 cm lateral to midline (lateral to the facet joints, depending on the thickness of the subcutaneous tissue) at the index level. Under fluoroscopic guidance, a Bovie cautery was used to carry the incision to the rib overlying the target area through the thoracolumbar fascia in a 45-degree oblique fashion medially aiming towards the costo-transverse and then ultimately towards the costo-vertebral junction at the index level. The METRx (Medtronic, Memphis, TN, USA) dilators were sequentially placed and then either a 16- or an 18-mm tubular working channels (5–8 cm long) were then passed over the dilator docking first on the transverse process. The operating microscope was used throughout the rest of the case. Once that lateral transverse process was resected, we advanced the working channel along the same axis towards the costo-vertebral junction and lateral pedicle (Figure 2A,2B). The medial rib head may need to be resected or displaced to allow advancement of the working channel.

Some pedicle and lateral vertebral body bones were removed to facilitate the tumor resection from within the vertebral body and epidural space, depending on tumor involvement, but the facets were usually left intact. Once all the soft tumor was removed (Figure 2C), if the resection cavity was not fairly well constrained, an Atlantis Vision (Medtronic, Memphis, TN, USA) 14–16 mm × 3.5 mm diameter screw was then placed into the contralateral cortical vertebral body, leaving the head about 6–8 mm proud, projecting into the resection cavity, but not past the posterior margin of the vertebral body (Figure 2D). At this point, poly-methyl methacrylate cement (Simplex-P, Stryker, Portage, MI, USA) was mixed, placed in small quantities consecutively into the cavity, and packed around the screw until it all hardened, so the cement was anchored by the screw without rotating or dislodging (Figure 2E). Once hemostasis was obtained, the working channel was removed (Figure 2F) and the skin was closed, and a simple dressing of Dermabond (J&J Ethicon, Raritan, NJ, USA) was applied and left on for a week or more. No subcutaneous drains were placed. Visualization of the placement of the screw is shown in Figure 3A-3D.
Control surgery
A midline incision was made from at least 4 levels above the target level, and at least 3 levels below the target level. The incision was carried further, dissecting the paraspinal muscles off the spinous processes and exposing the lamina, pars, and transverse processes bilaterally. Resection of the vertebral body tumor and epidural tumor in question was performed via a one (or more) of posterolateral approaches, including transpedicular vertebrectomy, costotransversectomy assisted vertebrectomy, or lateral extracavitary vertebrectomy, with or without laminectomy. After homeostasis was obtained, pedicle screw instrumentation was placed 2 or 3 levels above the resection level, and usually 2 below the target level, any available transverse process or facet bone surfaces were decorticated, a rod was placed bilaterally and secured, and some form of bone graft (allograft usually) was placed over the decorticated surfaces. The wounds were closed in a multilayered manner. Dressings and management of the drains and incisions were left to the individual surgeons.
Statistical analysis
Statistical analyses were performed using SAS software version 9.3 (SASv9.3, Cary, NC, USA). The specific statistical tests used include the Student’s t-test for nominal data with a normal distribution, the Mann-Whitney U test for nominal data with large variance, the Chi-squared test for categorical variables, and the Pearson correlation coefficient test as appropriate. The Kaplan-Meier analysis survival curve was used to generate 1-year survival. Two-sided P values were employed when applicable. Statistical significance was defined as P<0.05.
Results
Patient demographics
During the study period, 59 patients with tissue-proven diagnosis of cancer and radiographic evidence of MESCC underwent either MUST-D or standard open surgery before radiotherapy treatment. There were 21 (36%) patients in the MUST-D group and 38 (64%) in the control. There were no differences in baseline demographics between groups (Table 1). The mean age at the time of surgery was 59.7±11.1 years (P=0.62). Thirty patients (51%) were male and 29 (49%) were female (P=0.15). Twenty-six (44%) were White, 15 (25%) Black, 3 (5%) Asian, and 15 (25%) Other (P=0.42). There was equal representation between groups over time. Pathologies were similar between both groups. There was no significant difference in the distribution of lumbar, thoracic, and cervical incidence between groups (P=0.44). The mean anatomical center of the operation (the point of maximum compression or vertebral involvement) was T8/9, with a range of C5 to S1. The MUST-D group ranged from T2 to S1. The control group ranged from C5 to S1. The control group had a mean of 5.79±3.15 levels fused. The mean SINS scores were similar between groups (P=0.40). There was no significant difference between pre-operative focal cobb angle (MUST-D n=20; Control n=36; P=0.67). The MUST-D and control groups had similar mean preoperative HAI scores of 3.40±2.77 and 4.42±3.23 respectively (P=0.32).
Table 1
| Variables | MUST-D (n=21) | Control (n=38) | P value |
|---|---|---|---|
| Age (years) | 60.8±9.20 | 59.2±12.1 | 0.62 |
| Sex | 0.15 | ||
| Male | 8 [38] | 22 [58] | |
| Female | 13 [62] | 16 [42] | |
| Race | 0.42 | ||
| White | 11 [52] | 15 [39] | |
| Black | 4 [19] | 11 [29] | |
| Asian | 2 [10] | 1 [3] | |
| Other | 4 [19] | 11 [29] | |
| Representation over time | 0.38 | ||
| 2007–2011 | 13 [62] | 19 [50] | |
| 2012–2016 | 8 [38] | 19 [50] | |
| Histology | |||
| Breast | 6 | 7 | |
| Lung | 4 | 5 | |
| HCC | 7 | 6 | |
| GI (non-HCC) | 1 | 2 | |
| Myeloma | 1 | 4 | |
| Lymphoma | 1 | 2 | |
| Adrenal cortical carcinoma | 1 | 0 | |
| Renal | 0 | 5 | |
| Prostate | 0 | 3 | |
| Thyroid | 0 | 1 | |
| Uterine | 0 | 2 | |
| Unknown carcinoma | 0 | 1 | |
| Level of index | 0.44 | ||
| Cervical | 0 [0] | 1 [3] | |
| Thoracic | 16 [76] | 29 [76] | |
| Lumbar | 5 [24] | 8 [21] | |
| Preoperative SINS | 9.86±3.31 | 10.52±2.64 | 0.40 |
| Preoperative focal cobb angle (degrees) | 16.85±7.80 | 17.81±8.00 | 0.67 |
Values are presented as the mean ± standard deviation or number of patients as n [%] unless otherwise noted. MUST-D, Microscopically-Assisted Uninstrumented Spinal Tumor Decompression; GI, gastrointestinal; HCC, hepatocellular carcinoma; SINS, Spine Instability Neoplastic Score.
Intraoperative outcomes
The intraoperative outcomes are summarized in Table 2. The skin-to-closure time was shorter in the MUST-D group compared to the control group with a mean surgical time of 3.17±1.48 hours compared to 5.07±1.82 hours (P<0.001). The anesthesia duration was shorter in the MUST-D group compared to the control group, 5.43±1.85 vs. 7.20±2.09 hours (P=0.004). The MUST-D group trended towards lower EBL compared to the control group with 821.2±1,209 vs. 1,376±1,935 mL, respectively (P=0.06).
Table 2
| Variables | MUST-D (n=21) | Control (n=38) | P value |
|---|---|---|---|
| Intraoperative | |||
| Skin-to-closure time (hours) | 3.17±1.48 | 5.07±1.82 | <0.001* |
| Duration of anesthesia (hours) | 5.43±1.85 | 7.20±2.09 | 0.004* |
| Estimated blood loss (mL) | 821.2±1,209 | 1,376±1,935 | 0.06 |
| Perioperative | |||
| Length of stay (days) | 4.67±3.71 | 9.76±10.7 | 0.01* |
| Time to ambulation (days) | 0.41±0.87 | 3.68±5.62 | 0.02* |
| 30-day postoperative change in HAI† | –1.60 | +0.33 | 0.008* |
| Time until start of RTx (days) | 25.9±12.0 | 39.3±19.5 | 0.03* |
| 30-day postoperative complications | 0 [0] | 15 [39] | <0.001* |
| Long-term | |||
| Follow-up (years) | 2.80±3.68 | 1.76±2.97 | 0.24 |
| Recurrence at index level | 7 [33] | 10 [26] | 0.58 |
| Survival | |||
| 30-day mortality | 0 [0] | 2 [5.3] | 0.29 |
| 1-year mortality rate | 4 [19] | 14 [37] | 0.16 |
| Time until death (years) | 3.47±3.85 | 1.29±2.03 | 0.04* |
Values are presented as the mean ± standard deviation or number of patients as n [%] unless otherwise noted. †, HAI grade improvement (−), deterioration (+); *, statistical significance, P<0.05 (determined by the Student t-test). MUST-D, Microscopically-Assisted Uninstrumented Spinal Tumor Decompression; HAI, Hauser Ambulation Index; RTx, radiation therapy.
Perioperative outcomes
The mean length of hospital stay was shorter in the MUST-D group than in the control group, with 4.67±3.71 vs. 9.76±10.7 days (P=0.01). The MUST-D group had a shorter time to ambulation of 0.41±0.87 days compared to 3.68±5.62 days for the control group (P=0.02). There was a shorter time to initiation of adjuvant radiation treatment in the MUST-D group (n=14) of 25.9±12.0 days compared to the control group (n=19) of 39.3±19.5 days (P=0.03). No patients in the MUST-D group experienced serious complications or adverse events during the 30-day postoperative period compared to 15 (39%) patients in the control group (P<0.001). In the control group, 15 subjects experienced 16 events including 6 wound infections, 5 deep venous thromboses (DVTs), 4 pulmonary emboli, and 1 epidural hematoma (Table 2).
HAI ambulatory scores were evaluated at two different time points: pre-surgery and 30 days post-surgery. All pre-operative HAI scores were recorded, yet the 30-day data was available for 15 (71%) patients undergoing MUST-D and 24 (63%) of the control group patients. The MUST-D group displayed improved ambulation scores from 3.4 to 1.8 (–1.60, P=0.01) while the control group ambulation scores were unchanged 4.42 to 4.75 (+0.33, P=0.43) at 30-day post-operatively. The 30-day ambulation scores were significantly better for the MUST-D group compared to the control group (P=0.008) (Figure 4).

Long-term outcomes
In the MUST-D group, the postoperative Cobb angle decreased from a mean of 16.85±7.80 to 14.82±9.10 degrees with a mean follow-up of 1.34 years and a median of 1.17 years (n=17; P=0.01). In the control group, the postoperative Cobb angle decreased from a mean of 17.81±8.00 degrees to a mean of 14.22±5.18 degrees with a mean follow-up of 1.69 years post-op and a median of 1.63 years (n=17; P=0.02).
The MUST-D cohort had 7 (33%) cases of local recurrence. Of these patients, 2 underwent a repeat MUST-D procedure, 4 underwent a repeat decompression with instrumentation and fusion, and 1 opted for no repeat operation with a mean of 1.89±1.25 years after the original procedure. In the control group, 10 (26%) had local recurrence. Of these patients, 5 required revision fusion and 5 had no revision surgery with a mean of 2.14±1.64 years after the original procedure. There was no significant difference in recurrence (P=0.58) or reintervention between groups (P=0.56) (Table 2).
Within the MUST-D group, 12 (57%) patients died between surgery and the time of data collection vs. 20 (55%) in the control group (P=0.89). Of all patients that died, postoperative time to death was significantly longer in the MUST-D group (3.47±3.85 years) compared to the control group (1.29±2.03 years, P=0.04). There was no difference in 1-year mortality (P=0.16) or survival probability between groups as demonstrated by the Kaplan-Meier survival analysis (P=0.18) (Figure 5). Within 30 days post-operatively, 2 patients in the control group died, whereas no deaths occurred in the MUST-D group. In the control group, overall survival time was negatively correlated to the prevalence of complications within 30 days post-operatively (r=−0.384, P=0.02).

Discussion
In this study, we compared our proposed MIS VA technique to the standard open instrumented fusion approach for adequate decompression and stabilization of MESCC. Our findings indicate that the MUST-D approach results in improved intraoperative outcomes, fewer complications, and shorter hospital stays than standard open procedures. The MUST-D approach demonstrated improved short-term ambulation, as well as 3-year survival outcomes compared to the traditional approach.
There exists an extensive range of MIS techniques reported in the literature, with fluidity in what defines minimally invasive (26,27). The efficacy and outcomes of MIS techniques using non-percutaneous vertebral cement augmentation without extensive fixation compared to open approaches for tumor decompressions are largely underreported in the literature for patients with MESCC (19). Despite reports of complications from cement augmentation (28), including cement leakage and new fractures, none of the MUST-D patients in our study experienced any postoperative complications attributed to the use of VA. One reason may be related to the implementation of the anchoring screw used to stabilize the surrounding cement.
Intraoperative and perioperative outcomes
It is commonly reported in the literature that MIS approaches show significantly less blood loss (26,27,29-34). In terms of intraoperative outcomes, the MUST-D group demonstrated significantly shorter operative time and length of stay, which is consistent with other studies findings in MIS treatment of MESCC (27,29-34). In our study, while we saw a trend in lower blood loss in the MUST-D group compared to the control group (821.2±1,209 vs. 1,376±1,935 mL, respectively), this difference did not reach statistical significance. Two particular cases of well-vascularized tumors were noted within the MUST-D group.
Complications
Despite cancer patients’ heightened risk of infection due to their adjunctive therapy and underlying malignancy, no postoperative complications were observed in the MUST-D group compared to 15 patients in the control group (39%). Other studies have similarly found significantly fewer complications with MIS procedures compared to open procedures (26,32,33,35). Our overall complication rate in the open group (40–44%) was comparable to the literature (32,34). We believe the use of a tubular retractor, featuring a smaller paramedian incision and muscle splitting technique, may have contributed to this outcome. The paramedian location provides better tissue coverage over the surgical trajectory, potentially reducing incisional complications from midline adjuvant radiotherapy. Additionally, the smaller incision likely minimized the incisional exposure for potential complications. We suspect that the discrepancy in major medical complications between the two approaches is likely related to the aforementioned intraoperative differences. Factors such as blood loss and duration of anesthesia exposure in the prone position often serve as independent predictors of postoperative complications. In addition, as supported by Kumar et al. and their results comparing MIS with open surgery for metastatic spine tumors, we also hypothesize that the lack of postoperative complications in the MUST-D group helped facilitate shorter times to adjuvant treatment compared to the control (36).
Ambulation
In our study, the MUST-D group displayed significantly faster time to ambulation (MUST-D, 0.41±0.87 days vs. control, 3.68±5.62 days, P=0.02). These findings are consistent with other literature demonstrating that MIS procedures lead to faster ambulatory initiation compared to traditional open surgery (29,37). In documenting the HAI ambulation scores to assess patient mobility, we observed no differences in baseline ambulation between the two groups. However, postoperatively, the MUST-D group displayed an improvement in HAI scores, while the control group slightly worsened. Conversely, the majority of the literature has found that MIS and open surgery are comparable in terms of functional outcomes (20,26,32,38). This discrepancy in findings may be due to the existing broad variation of MIS approaches. A majority of MIS approaches still utilize instrumentation for stabilization compared to our un-instrumented approach. In addition, there is a wide variation in how ambulatory status is defined and measured, including the use of several scoring systems (e.g., Karnofsky score, Frankel score, ASIA impairment score) (39). In our study, we chose to utilize the HAI because of its incorporation of a timed walking (24,40). Our finding of improved mobility in the MUST-D group contributes to an improved post-operative quality of life. We hypothesize the small incision, muscle splitting, and un-instrumented technique used in MUST-D resulted in less incisional and muscle dissection-related pain, facilitating quicker mobility.
Time to RTx
Within the analysis of adjuvant radiotherapy, other studies have found that MIS surgery for MESCC had a shorter time to start adjuvant RTx (36,41). Our results corroborate these findings that patients who underwent MUST-D surgery had a significantly faster time to radiotherapy compared to open surgery (25.9±12.0 vs. 39.3±20.8 days; P=0.03). Due to the variation in primary cancers in our patient population and limited medical records, we decided not to include further discrepancies between chemotherapy and immunotherapy in our analysis.
Long-term outcomes
Cobb angle
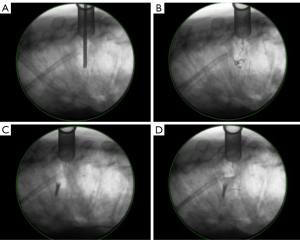
One of the critiques within the literature regarding un-instrumented techniques for MESCC decompression is the potential for long-term destabilization without adequate instrumentation. Despite these concerns, studies such as Deutsch et al. have demonstrated successful MIS MESCC decompression without both instrumentation or VA and no signs of postoperative instability or kyphosis, albeit being a small case series (22). Our study expands this to a larger patient population and incorporates cement augmentation as a proxy for standard instrumented fusion. There was no observable difference in preoperative Cobb angles between groups and both groups saw a subsequent decrease in Cobb angle postoperatively with no new focal instability within the MUST-D cohort specifically (Figure 6A-6D).

Survival
In our study, we found comparable mortality rates between MUST-D and the control group, which is expected given that there are no differences across primary tumor demographics. The 1-year Kaplan-Meier analysis did not demonstrate a difference in survival probability between groups (Figure 5). However, we observed a significantly longer time to death within the MUST-D group. Additionally, within the control group, survival was shown to be negatively correlated to complications. Paulino Pereira et al. similarly found, in a study of 647 patients with MESCC, that 30-day complications were associated with worsened survival (42). Given the prevalence of complication rates, slower time to RTx, and 2 patient deaths within the 30-day post-operative period in the control group, we propose that post-operative comorbidities may contribute to the shorter duration of survival observed within the open procedure approach. Survival after surgery for metastatic disease has been predicted by various methods, including the Tomita and Tokuhashi scales, and our previously published system, the Jenkins Survival Index (JSI) (43,44). Despite this, the estimation of survival is very difficult and is largely influenced by the primary histology, the extent of tumor burden, and the patient’s general condition (36). Nonetheless, MIS surgical treatments, such as the MUST-D approach, can still provide meaningful palliative benefits in the short term.
Limitations
Limitations of this study include that this was a retrospective study performed at a single academic medical center with a relatively small sample patient population. This was an unblinded study in which the surgeon uniformly offered and operated on all patients whom a MUST-D procedure was possible. Upon shared decision-making of risks, benefits, and alternatives, all patients opted for this approach. Some limitations are inherent to a retrospective cohort design including incomplete data. For example, standing radiographs were largely unobtainable due to severe neurological dysfunction, functional limitations, and lack of patient follow-up. As a result, we opted to review available CT and MRI imaging for consistent supine radiological assessment of the Cobb angle analysis. While the SINS scores were comparable between groups, there was still some variation in the tumor burden and index location, in which patients with cervical, thoracic, and lumbar cases were analyzed together. While this approach may be more suitable for one region than another, such distinctions have not been investigated thus far. Additionally, in reviewing multiple surgeons, we did not have uniform standardized PROMs. Future studies would address this shortcoming in a prospective, multi-center format. Given the lack of surgical hardware, fewer days of hospital stay, and shorter operative time shown in our study, we predict significant cost savings to both the patient and hospital system. Future studies should be performed to assess the cost-benefit analysis in utilizing the MUST-D technique for MESCC treatment.
Conclusions
The MUST-D minimally invasive surgical approach offers multiple potential advantages compared to the open surgical approach in the treatment of symptomatic metastatic epidural spinal disease. Our study demonstrates improved perioperative variables including length of operation, duration of anesthesia, hospital stay, and fewer complications in the MUST-D group. Although there were no differences in 1-year mortality rate or survival, the MUST-D group showed significantly faster initiation of adjunctive RTx and longer time to death compared to patients with open procedures. Mobility was also better in the MUST-D group both in time to ambulation and 30-day HAI score postoperatively. Further, there was no evidence of long-term postoperative destabilization via Cobb angle or any differences in revision rates observed between the MUST-D and open cohort. These findings further warrant strong consideration of the MUST-D surgical approach as an initial treatment modality for MESCC management. Prospective, randomized studies with protocols to standardize long-term radiographic and clinical outcomes are indicated to corroborate the benefits of the MUST-D surgical technique over open procedures in patients with MESCC.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-24-135/rc
Data Sharing Statement: Available at https://jss.amegroups.com/article/view/10.21037/jss-24-135/dss
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-24-135/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-24-135/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was determined to be exempt from IRB oversight initially by the Icahn School of Medicine at Mount Sinai until expiration, and then by Advarra for the remainder of the study period. Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Aaron AD. The management of cancer metastatic to bone. JAMA 1994;272:1206-9. [Crossref] [PubMed]
- Black P. Spinal metastasis: current status and recommended guidelines for management. Neurosurgery 1979;5:726-46. [Crossref] [PubMed]
- Sciubba DM, Petteys RJ, Dekutoski MB, et al. Diagnosis and management of metastatic spine disease. A review. J Neurosurg Spine 2010;13:94-108. [Crossref] [PubMed]
- Traul DE, Shaffrey ME, Schiff D. Part I: spinal-cord neoplasms-intradural neoplasms. Lancet Oncol 2007;8:35-45. [Crossref] [PubMed]
- Miscusi M, Polli FM, Forcato S, et al. Comparison of minimally invasive surgery with standard open surgery for vertebral thoracic metastases causing acute myelopathy in patients with short- or mid-term life expectancy: surgical technique and early clinical results. J Neurosurg Spine 2015;22:518-25. [Crossref] [PubMed]
- Husain ZA, Sahgal A, Chang EL, et al. Modern approaches to the management of metastatic epidural spinal cord compression. CNS Oncol 2017;6:231-41. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366:643-8. [Crossref] [PubMed]
- Findlay GF. The role of vertebral body collapse in the management of malignant spinal cord compression. J Neurol Neurosurg Psychiatry 1987;50:151-4. [Crossref] [PubMed]
- Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 2001;27:165-76. [Crossref] [PubMed]
- Balabhadra S, Kuban JD, Lee S, et al. Association of Inferior Vena Cava Filter Placement With Rates of Pulmonary Embolism in Patients With Cancer and Acute Lower Extremity Deep Venous Thrombosis. JAMA Netw Open 2020;3:e2011079. [Crossref] [PubMed]
- Rognoni E, Watt FM. Skin Cell Heterogeneity in Development, Wound Healing, and Cancer. Trends Cell Biol 2018;28:709-22. [Crossref] [PubMed]
- Mueller K, Zhao D, Johnson O, et al. The Difference in Surgical Site Infection Rates Between Open and Minimally Invasive Spine Surgery for Degenerative Lumbar Pathology: A Retrospective Single Center Experience of 1442 Cases. Oper Neurosurg (Hagerstown) 2019;16:750-5. [Crossref] [PubMed]
- Tibbs MK. Wound healing following radiation therapy: a review. Radiother Oncol 1997;42:99-106. [Crossref] [PubMed]
- Quraishi NA, Ahmed MS, Arealis G, et al. Does surgical site infection influence neurological outcome and survival in patients undergoing surgery for metastatic spinal cord compression? Eur Spine J 2019;28:792-7. [Crossref] [PubMed]
- Gong Y, Zhuang H, Chong S, et al. Delayed postoperative radiotherapy increases the incidence of radiographic local tumor progression before radiotherapy and leads to poor prognosis in spinal metastases. Radiat Oncol 2021;16:21. [Crossref] [PubMed]
- Park S, Park JW, Park JH, et al. Factors affecting the prognosis of recovery of motor power and ambulatory function after surgery for metastatic epidural spinal cord compression. Neurosurg Focus 2022;53:E11. [Crossref] [PubMed]
- Barzilai O, Bilsky MH, Laufer I. The Role of Minimal Access Surgery in the Treatment of Spinal Metastatic Tumors. Global Spine J 2020;10:79S-87S. [Crossref] [PubMed]
- Laufer I, Bilsky MH. Advances in the treatment of metastatic spine tumors: the future is not what it used to be. J Neurosurg Spine 2019;30:299-307. [Crossref] [PubMed]
- Molina CA, Gokaslan ZL, Sciubba DM. Diagnosis and management of metastatic cervical spine tumors. Orthop Clin North Am 2012;43:75-87. viii-ix. [Crossref] [PubMed]
- Saadeh YS, Elswick CM, Fateh JA, et al. Analysis of Outcomes Between Traditional Open versus Mini-Open Approach in Surgical Treatment of Spinal Metastasis. World Neurosurg 2019;130:e467-74. [Crossref] [PubMed]
- Fessler RG, O’Toole JE, Eichholz KM, et al. The development of minimally invasive spine surgery. Neurosurg Clin N Am 2006;17:401-9. [Crossref] [PubMed]
- Deutsch H, Boco T, Lobel J. Minimally invasive transpedicular vertebrectomy for metastatic disease to the thoracic spine. J Spinal Disord Tech 2008;21:101-5. [Crossref] [PubMed]
- Weller SJ, Rossitch E Jr. Unilateral posterolateral decompression without stabilization for neurological palliation of symptomatic spinal metastasis in debilitated patients. J Neurosurg 1995;82:739-44. [Crossref] [PubMed]
- Hauser SL, Dawson DM, Lehrich JR, et al. Intensive immunosuppression in progressive multiple sclerosis. A randomized, three-arm study of high-dose intravenous cyclophosphamide, plasma exchange, and ACTH. N Engl J Med 1983;308:173-80. [Crossref] [PubMed]
- Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976) 2010;35:E1221-9. [Crossref] [PubMed]
- Alshareef M, Klapthor G, Alawieh A, et al. Evaluation of open and minimally invasive spinal surgery for the treatment of thoracolumbar metastatic epidural spinal cord compression: a systematic review. Eur Spine J 2021;30:2906-14. [Crossref] [PubMed]
- Schupper AJ, Patel S, Steinberger JM, et al. The role of minimally invasive surgery within a multidisciplinary approach for patients with metastatic spine disease over a decade: A systematic review. Neuro Oncol 2024;26:417-28. [Crossref] [PubMed]
- Hulme PA, Krebs J, Ferguson SJ, et al. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine (Phila Pa 1976) 2006;31:1983-2001. [Crossref] [PubMed]
- Park Y, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine (Phila Pa 1976) 2007;32:537-43. [Crossref] [PubMed]
- Sidhu GS, Henkelman E, Vaccaro AR, et al. Minimally invasive versus open posterior lumbar interbody fusion: a systematic review. Clin Orthop Relat Res 2014;472:1792-9. [Crossref] [PubMed]
- Hinojosa-Gonzalez DE, Roblesgil-Medrano A, Villarreal-Espinosa JB, et al. Minimally Invasive versus Open Surgery for Spinal Metastasis: A Systematic Review and Meta-Analysis. Asian Spine J 2022;16:583-97. [Crossref] [PubMed]
- Hikata T, Isogai N, Shiono Y, et al. A Retrospective Cohort Study Comparing the Safety and Efficacy of Minimally Invasive Versus Open Surgical Techniques in the Treatment of Spinal Metastases. Clin Spine Surg 2017;30:E1082-7. [Crossref] [PubMed]
- Pennington Z, Ahmed AK, Molina CA, et al. Minimally invasive versus conventional spine surgery for vertebral metastases: a systematic review of the evidence. Ann Transl Med 2018;6:103. [Crossref] [PubMed]
- Hansen-Algenstaedt N, Kwan MK, Algenstaedt P, et al. Comparison Between Minimally Invasive Surgery and Conventional Open Surgery for Patients With Spinal Metastasis: A Prospective Propensity Score-Matched Study. Spine (Phila Pa 1976) 2017;42:789-97. [Crossref] [PubMed]
- Pranata R, Lim MA, Vania R, et al. Minimal Invasive Surgery Instrumented Fusion versus Conventional Open Surgical Instrumented Fusion for the Treatment of Spinal Metastases: A Systematic Review and Meta-analysis. World Neurosurg 2021;148:e264-74. [Crossref] [PubMed]
- Kumar N, Malhotra R, Maharajan K, et al. Metastatic Spine Tumor Surgery: A Comparative Study of Minimally Invasive Approach Using Percutaneous Pedicle Screws Fixation Versus Open Approach. Clin Spine Surg 2017;30:E1015-21. [Crossref] [PubMed]
- Pereira P, Buzek D, Franke J, et al. Surgical data and early postoperative outcomes after minimally invasive lumbar interbody fusion: results of a prospective, multicenter, observational data-monitored study. PLoS One 2015;10:e0122312. [Crossref] [PubMed]
- Kumar N, Malhotra R, Zaw AS, et al. Evolution in treatment strategy for metastatic spine disease: Presently evolving modalities. Eur J Surg Oncol 2017;43:1784-801. [Crossref] [PubMed]
- Nguyen L, Agaronnik N, Ferrone ML, et al. Evaluating ambulatory function as an outcome following treatment for spinal metastases: a systematic review. Spine J 2021;21:1430-9. [Crossref] [PubMed]
- Bartolo M, Zucchella C, Pace A, et al. Early rehabilitation after surgery improves functional outcome in inpatients with brain tumours. J Neurooncol 2012;107:537-44. [Crossref] [PubMed]
- Echt M, Stock A, De la Garza Ramos R, et al. Separation surgery for metastatic epidural spinal cord compression: comparison of a minimally invasive versus open approach. Neurosurg Focus 2021;50:E10. [Crossref] [PubMed]
- Paulino Pereira NR, Ogink PT, Groot OQ, et al. Complications and reoperations after surgery for 647 patients with spine metastatic disease. Spine J 2019;19:144-56. [Crossref] [PubMed]
- Wei D, Nistal DA, Sobotka S, et al. New Predictive Index for Survival in Symptomatic Spinal Metastases. World Neurosurg 2019;123:e133-40. [Crossref] [PubMed]
- Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001;26:298-306. [Crossref] [PubMed]


