Functional and survival outcomes in patients undergoing surgical treatment for metastatic disease of the spine
Introduction
Strategies and treatment modalities for cancer have significantly improved in the past few decades and have resulted in tremendous improvements in overall survival (OS) rates for such patients. Unfortunately, spinal metastasis has been noted in up to 40% of cancer patients (1,2). Additionally, as survival lengths continue to increase, the incidence of such spinal metastasis will only continue to increase (3) —more than 18,000 new cases are reported every year in North America alone (4). Additionally, post mortem examinations have revealed the incidence of spinal metastasis to be much higher (5).
Prognostic tools have continued to evolve as oncologic treatments and surgical modalities continue to improve (6-8). However, with survival rates that have been reported to be anywhere from a few months to a few years at best (9,10), it is imperative to evaluate patients’ functional status and describe how surgical intervention can affect this aspect of their quality of life following diagnosis and treatment.
In this study, we sought to explore the functional and survival outcomes of patients with metastatic disease to the spine that undergoes operative treatment. Specifically, our primary goal was to assess patients’ functional status via means of their ambulatory status prior to, after their surgical intervention and at the end of their disease course. Additionally, our secondary goal was to analyze prognostic factors for survival in addition to assessing survival times.
Methods
After obtaining Institutional Review Board (IRB) approval, a retrospective cohort study of a prospective database at a major cancer center was conducted to assess the functional and survival outcomes of patients undergoing surgical treatment for metastatic disease of their spine.
Patients undergoing surgical treatment at our institution by the senior author between January 2010 and December 2015 were identified based on a retrospective review of a prospective study and were considered for the study. This time period was chosen to achieve a minimum of 6 months follow-up. Patients were excluded from the study if they were younger than 18 years of age and if they underwent non-surgical treatment for their metastatic disease to their spine. Additionally, primary tumors of the spine undergoing surgical treatment were also excluded.
Of a total of 85 patients identified, 55 met the inclusion criteria. Patient demographics and tumor characteristics were collected, including age, gender, race, smoking status, primary malignancy type, number of vertebral levels affected, i.e., the extent of the metastasis, and location of their spinal metastasis. The presence or absence of pain and any neurological deficits were also collected. Patients’ ambulatory status was collected at four time points; initial presentation at index visit, immediate preoperative state, first clinic visit post-operatively, and at last follow-up visit. Ambulatory status was analyzed as a binary variable—patients were categorized as either being able to ambulate, with or without the use of assistive devices such as a cane, or being unable to ambulate (i.e., being wheelchair or bed bound). Additionally, patient’s American Spinal Injury Association (ASIA) (11) impairment score at time of their initial clinical visit and Eastern Cooperative Oncology Group (ECOG) score at the time of their last follow-up appointment were also collected. The ECOG performance status is a scale used to assess how a patient’s disease is progressing, assess how the disease affects the daily living abilities of the patient, and determine appropriate treatment and prognosis (12,13).
OS was measured in two ways: (I) from the date of diagnosis of spinal metastasis to date of death from any cause (OS1); and (II) from the date of surgical treatment for metastatic disease to the date of death from any cause (OS2). Surviving patients were censored at the date of last follow-up. For those whose spinal metastases were identified after diagnosis of primary malignancy (more than 14 days between initial cancer diagnosis date and spinal metastasis diagnosis date), time to diagnosis of spinal metastases was measured from the date of diagnosis of primary malignancy to date of diagnosis of spinal metastases. No censoring was required for time to spinal metastases as all patients included in the analyses were eventually diagnosed with spinal metastases.
Frequency and proportion of patient and tumor characteristics were summarized for categorical variables and descriptive statistics were used to summarize continuous data. Ambulatory status was compared before and after surgery using McNemar’s tests. Kaplan-Meier techniques were used to estimate survival distributions for each of the time to event endpoints. Univariate and multivariate Cox proportional hazards regression assessed the impact of patient and disease characteristics on OS and were used to determine hazard ratios (HR) to estimate the magnitude of the impact of those factors on survival. Multivariate Cox models were determined for the outcome of OS (measured from date of spinal metastasis diagnosis, OS1) using backward elimination and forward selection modeling procedures (significance levels of P=0.10). Individual prognostic factors were identified through univariate Cox models for all potential covariates (age, race, gender, smoking status, primary malignancy type, spine burden, pre-operative ambulatory status, presence of pain, radiotherapy, chemotherapy, and presence of extra-spinal metastases).
Statistical software SAS (version 9.4) was used for all data analysis. Unless otherwise noted, a 2-sided α=0.05 significance level was used.
Results
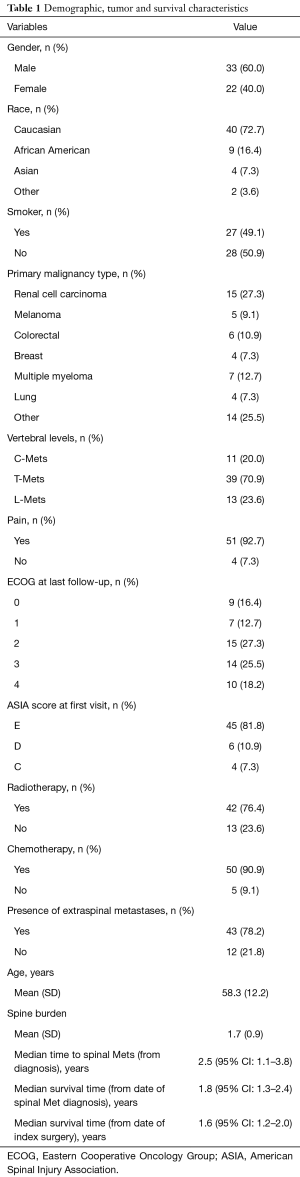
Of the total of 85 patients that were operated by the senior surgeon in the group from January 2010 to December 2015, 55 patients met inclusion criteria (Table 1). Approximately 60% of the patients were males and the majority of patients (72.7%) were of Caucasian origin. Nearly half of the study population included prior or current smokers (49.1%). In analyzing primary malignancy types, renal cell carcinoma (RCC) was the most commonly identified tumor type at our institution (27.3%). Additionally, multiple myeloma, colorectal and melanoma cancer types also composed a sizable portion of our study population (9.1–12.7% each). The “Other” category (25.5%) included squamous cell carcinoma of the ear, nose and throat, prostate, bladder and testicular cancer—groups with too few numbers individually to allow for any meaningful statistical analysis.
Full table
Most patients (92.7%, 51 of 55 patients) reported pain as one of the chief complaints upon their initial presentation and diagnosis of their spinal metastasis. Nonetheless, nearly 81.8% (45 of the 55 patients) had ASIA score of E—signifying a normal neurological exam upon initial clinical presentation. In reviewing tumor predilection to various regions of the spine, similar to prior studies, we found that the majority of the metastasis was at the thoracic spine level (70.9%) followed by the lumbar spine (23.6%) and the cervical spine (20.0%) (14). Additionally, it was found that metastasis involved an average of 1.7 vertebral levels. Forty-three of the patients in our cohort (78.2%) also had extra-spinal metastasis in addition to their primary tumor location. Forty-two patients (76.4%) in study cohort received neo-adjuvant and/or adjuvant radiotherapy. Similarly, 90.9% received adjuvant chemotherapy (Table 2).

Full table
At the time of initial consultation, 90.9% of the patients were either independently ambulatory or ambulatory using assistive devices. This proportion had decreased slightly to 87.3% immediately prior to surgical intervention. Post-surgery analysis revealed that only 3.6% of the patients were unable to ambulate either independently or with assistive devices after their surgical intervention at the first post-operative visit with 96.4% regaining ability to ambulate. A McNemar’s test indicated that the ambulatory rates were significantly different before and after surgical intervention (P=0.0253). However, by the time of their final follow-up visit, nearly 36.4% of the patients were wheelchair-bound or on bed rest. Only 63.6% patients were able to ambulate either independently or with the use of assistive devices at their last follow-up—a drastic decrease from the 96.4% seen at the time of the first post-operative visit (P<0.0001) (Table 2).
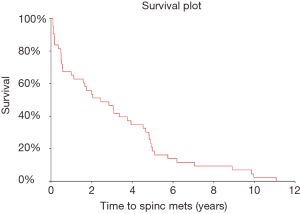
After excluding 12 patients whose spinal metastases were identified at the time of or within 2 weeks of their initial cancer diagnosis, the time to spinal metastases distribution was estimated (Figures 1-3) and it was found that the median time to spinal metastases was 2.5 years (95% CI: 1.1–3.8 years).
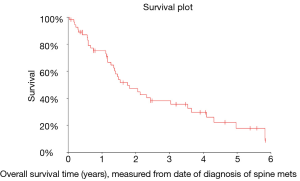
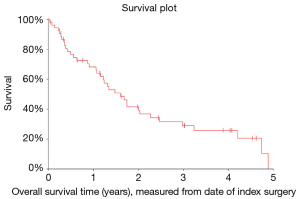
In analyzing OS measured from diagnosis of spinal metastasis to death (OS1), it was found that the median OS was 1.8 years (95% CI: 1.3–2.4 years). Similarly, OS measured from surgical intervention to death (OS2) was also analyzed and it was found that the median OS was 1.6 years (95% CI: 1.2–2.0 years).
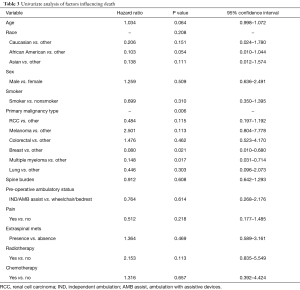
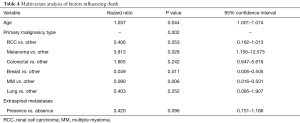
Univariate and multivariate modeling procedures identified variables that could impact OS. Table 3 includes all of the results of the variables evaluated in univariate Cox models for OS. Of the prognostic factors evaluated, age (P=0.064) and primary malignancy type (P=0.006) were found to be individually associated with survival. Table 4 contains the multivariate Cox regression results for OS. After model selection procedures, age, primary malignancy type, and presence extra-spinal metastases were included in the final model for OS. The effect of age on survival is significant (P=0.044), even after adjusting for primary malignancy type and presence/absence of extra-spinal metastases, with increasing age associated with increased risk of death (HR: 1.037; 95% CI: 1.001–1.074). Also, the effect of primary malignancy type on survival is significant (P=0.002) after adjusting for the subject’s age and presence/absence of extra-spinal metastases. The risk of death in subjects with melanoma or colorectal cancer was higher compared to those with “Other” classification (HR: 3.813 and 1.905, respectively). Additionally, presence of extra-spinal metastases does not appear to increase the risk of death, (HR: 0.420; Presence vs. Absence, 95% CI: 0.151–1.166; P=0.096), after adjusting for age and primary malignancy type.
Full table
Full table
Discussion
Symptomatic metastasis to the vertebral column has been found in 10% of patients with cancer (15,16). Surgery has been shown to offer a reliable treatment modality (17). Surgical techniques have improved including obtaining circumferential spinal cord decompression and improved stabilization (18). However, not much is currently described regarding functional status, mainly ambulatory status, following surgical intervention and over the patient’s disease course. Additionally, survival outcomes were also evaluated.
In this study, 91% of the study population was able to independently ambulate or with the use of an assistive device at the time of initial consultation. This proportion was the greatest in the immediate postoperative visit at 96.4%. However, at the time of the final follow-up, the proportion that was able to ambulate either independently or with assistance was the lowest at 63.7%. Changes in ambulatory status can sometimes be the presenting sign of spinal metastasis and epidural compression; this can present as a slow, progressive onset or rapid neurologic emergency (19). Additionally, preoperative ability to ambulate has been prognostically related to maintaining post-operative ambulation (20,21). Surgical intervention has also been noted to allow for the gain of ambulation in patients who were previously non-ambulatory prior to surgery (22). However, high complication rates have been reported in such patients for whom surgical intervention was offered to improve their ambulatory status (22).
As evidenced by the results of our study, long-term ambulatory status in this patient population declines rather precipitously. While only 3.6% of the patients were wheelchair or bed bound at the first postoperative visit, around 36.4% of the patients in the study were unable to ambulate at the last follow-up visit. While surgery has the potential to maintain or improve the ambulatory status post-operatively, the long-term prognosis for ambulation is rather grim. For example, Patchell et al. found that surgical intervention allowed such patients the ability to walk for a median of 122 days—a significant improvement over the non-surgical group (17). Nonetheless, this post-surgical improvement in ambulation is difficult to be sustained over the patient’s disease course. Decreases in overall performance statuses and overall disease progression appear to be the driving force in curtailing ambulation as the patient moves forward in their disease course (23). Thus, it is important that prior to surgical intervention, patients should be counseled on the risks of not being able to maintain their ambulatory status particularly at the tail end of their disease course.
Survival analysis showed that the median time from cancer diagnosis to spinal metastasis was 2.5 years while the time from surgical intervention to death was 1.6 years. Surgical techniques and overall treatment modalities have improved in the past few decades—however, despite these improvements, as described above and, similar to other studies, OS still remains grim (9,24).
Additionally, when analyzing prognostic factors, our analysis revealed that increasing age and certain subtypes such as melanoma might lead to increased odds of death while multiple myeloma has improved prognosis compared to other subtypes. This has been reported in other studies with tumor type as a risk factor influencing survival (25,26). The biology of the tumor and its inherent aggressiveness can have an effect on obtaining both local-control at the site of spinal metastasis as well as OS. Melanoma, in particular, has been associated with increased local recurrence rates in the spine (27). Additionally, the radiosensitivity of the tumor can also have an impact on affecting survival through the use of adjuvant radiotherapy. For example, hematopoietic tumors such as lymphomas, multiple myeloma, as well as prostate, breast, small cell lung and germ cell tumors are more receptive to radiotherapy and can be a useful adjunct in obtaining spinal disease control (28,29).
Advanced age while not a strict contraindication against surgery does have risks associated other than lower expected OS. Decreased biological reserves can place elderly patients at an increased risk for perioperative complications (30). Nonetheless, surgery can often provide an important palliation producing procedure that can significantly improve the quality of life in their remaining years.
In our study, the presence of extra-spinal metastasis was not found to increase the risk of death. This is in contrast to findings reported and used in scoring systems such as the Tomita and Tokuhashi scoring systems (8,31). This raises the possibility that extra-spinal metastasis may not always serve as a strong contraindication against surgical intervention and that a thorough discussion should be had with patients regarding their expectations and the likely outcomes that will result from surgical intervention.
In conclusion, functional outcomes are maintained or improved in the postoperative period. However, patients should be made aware of the chance of loss of their ambulatory status and the possibility of being either wheelchair or bed bound as their disease course progresses. Additionally, survival outcomes despite improvements in treatment modalities still remain modest.
This was a single institution study that primarily attempts to characterize the ambulatory status of patients undergoing surgical intervention for metastatic spine disease at various points in their disease course with survival outcomes as a secondary outcome analysis—it is thus not representative of findings elsewhere. Nonetheless, the relations and conclusions derived remain highly probable. Additional studies evaluating the ambulatory status are needed to further firmly establish these findings in larger numbers.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The research project has been approved by a suitably constituted Ethics Committee of the institution within which the work was undertaken and that it conforms to the provisions of the Declaration of Helsinki (as revised in Edinburgh 2000).
References
- Fornasier VL, Horne JG. Metastases to the vertebral column. Cancer 1975;36:590-4. [Crossref] [PubMed]
- Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer 1950;3:74-85. [Crossref] [PubMed]
- Choi D, Crockard A, Bunger C, et al. Review of metastatic spine tumour classification and indications for surgery: the consensus statement of the Global Spine Tumour Study Group. Eur Spine J 2010;19:215-22. [Crossref] [PubMed]
- Gokaslan ZL, York JE, Walsh GL, et al. Transthoracic vertebrectomy for metastatic spinal tumors. J Neurosurg 1998;89:599-609. [Crossref] [PubMed]
- Hatrick NC, Lucas JD, Timothy AR, et al. The surgical treatment of metastatic disease of the spine. Radiother Oncol 2000;56:335-9. [Crossref] [PubMed]
- Tokuhashi Y, Matsuzaki H, Toriyama S, et al. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 1990;15:1110-3. [Crossref] [PubMed]
- Wang M, Bunger CE, Li H, et al. Predictive value of Tokuhashi scoring systems in spinal metastases, focusing on various primary tumor groups: evaluation of 448 patients in the Aarhus spinal metastases database. Spine (Phila Pa 1976) 2012;37:573-82. [Crossref] [PubMed]
- Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001;26:298-306. [Crossref] [PubMed]
- Tatsui CE, Suki D, Rao G, et al. Factors affecting survival in 267 consecutive patients undergoing surgery for spinal metastasis from renal cell carcinoma. J Neurosurg Spine 2014;20:108-16. [Crossref] [PubMed]
- Chong S, Shin SH, Yoo H, et al. Single-stage posterior decompression and stabilization for metastasis of the thoracic spine: prognostic factors for functional outcome and patients' survival. Spine J 2012;12:1083-92. [Crossref] [PubMed]
- El Masry WS, Tsubo M, Katoh S, et al. Validation of the American Spinal Injury Association (ASIA) motor score and the National Acute Spinal Cord Injury Study (NASCIS) motor score. Spine (Phila Pa 1976) 1996;21:614-9. [Crossref] [PubMed]
- Young J, Badgery-Parker T, Dobbins T, et al. Comparison of ECOG/WHO performance status and ASA score as a measure of functional status. J Pain Symptom Manage 2015;49:258-64. [Crossref] [PubMed]
- O'Mahony S, Nathan S, Mohajer R, et al. Survival Prediction in Ambulatory Patients With Stage III/IV Non-Small Cell Lung Cancer Using the Palliative Performance Scale, ECOG, and Lung Cancer Symptom Scale. Am J Hosp Palliat Care 2016;33:374-80. [Crossref] [PubMed]
- Constans JP, de Divitiis E, Donzelli R, et al. Spinal metastases with neurological manifestations. Review of 600 cases. J Neurosurg 1983;59:111-8. [Crossref] [PubMed]
- Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer 1997;80:1614-27. [Crossref] [PubMed]
- Sundaresan N, Digiacinto GV, Hughes JE, et al. Treatment of neoplastic spinal cord compression: results of a prospective study. Neurosurgery 1991;29:645-50. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366:643-8. [Crossref] [PubMed]
- Quraishi NA, Rajabian A, Spencer A, et al. Reoperation rates in the surgical treatment of spinal metastases. Spine J 2015;15:S37-43. [Crossref] [PubMed]
- Gerszten PC, Welch WC. Current surgical management of metastatic spinal disease. Oncology (Williston Park) 2000;14:1013-24; discussion 1024, 29-30.
- Park JH, Jeon SR. Pre- and postoperative lower extremity motor power and ambulatory status of patients with spinal cord compression due to a metastatic spinal tumor. Spine (Phila Pa 1976) 2013;38:E798-802. [Crossref] [PubMed]
- Chaichana KL, Woodworth GF, Sciubba DM, et al. Predictors of ambulatory function after decompressive surgery for metastatic epidural spinal cord compression. Neurosurgery 2008;62:683-92; discussion 683-92. [Crossref] [PubMed]
- Kim CH, Chung CK, Jahng TA, et al. Resumption of ambulatory status after surgery for nonambulatory patients with epidural spinal metastasis. Spine J 2011;11:1015-23. [Crossref] [PubMed]
- Metastatic Spinal Cord Compression: Diagnosis and Management of Patients at Risk of or with Metastatic Spinal Cord Compression. Cardiff (UK): National Collaborating Centre for Cancer, 2008.
- Sellin JN, Suki D, Harsh V, et al. Factors affecting survival in 43 consecutive patients after surgery for spinal metastases from thyroid carcinoma. J Neurosurg Spine 2015;23:419-28. [Crossref] [PubMed]
- Tokuhashi Y, Matsuzaki H, Oda H, et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30:2186-91. [Crossref] [PubMed]
- Bollen L, de Ruiter GC, Pondaag W, et al. Risk factors for survival of 106 surgically treated patients with symptomatic spinal epidural metastases. Eur Spine J 2013;22:1408-16. [Crossref] [PubMed]
- Lau D, Than KD, La Marca F, et al. Independent predictors for local recurrence following surgery for spinal metastasis. Acta Neurochir (Wien) 2014;156:277-82. [Crossref] [PubMed]
- Maranzano E, Bellavita R, Rossi R, et al. Short-course versus split-course radiotherapy in metastatic spinal cord compression: results of a phase III, randomized, multicenter trial. J Clin Oncol 2005;23:3358-65. [Crossref] [PubMed]
- Rades D, Fehlauer F, Schulte R, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol 2006;24:3388-93. [Crossref] [PubMed]
- Jalai CM, Worley N, Marascalchi BJ, et al. The Impact of Advanced Age on Peri-Operative Outcomes in the Surgical Treatment of Cervical Spondylotic Myelopathy: A Nationwide Study Between 2001 and 2010. Spine (Phila Pa 1976) 2016;41:E139-47. [Crossref] [PubMed]
- Tokuhashi Y, Uei H, Oshima M, et al. Scoring system for prediction of metastatic spine tumor prognosis. World J Orthop 2014;5:262-71. [Crossref] [PubMed]